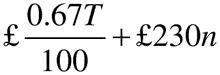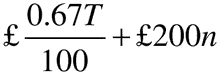- Latest available (Revised)
- Original (As made)
The Veterinary Medicines Regulations 2013
You are here:
- UK Statutory Instruments
- 2013 No. 2033
- SCHEDULE 7
More Resources
Status:
This is the original version (as it was originally made).
Regulation 16
SCHEDULE 7Fees
PART 1 Introduction
1.Interpretation
2.Payment of fees
3.Time of payment
4.Multiple inspections
5.Expenses for inspections outside the United Kingdom
6.Translation
PART 2 Fees relating to marketing authorisations
7.Specified pharmaceutical applications
8.Decentralised pharmaceutical application where the United Kingdom is the reference member State
9.Application for a marketing authorisation for an immunological or biosimilar product
10.Decentralised immunological application where the United Kingdom is the reference member State
11.Applications for a marketing authorisation using data already assessed
12.Application for an exceptional marketing authorisation (pharmaceutical)
13.Fees for an application for an exceptional marketing authorisation (immunological)
14.Fee for the conversion from an exceptional to a full marketing authorisation
15.Application for a marketing authorisation relating to a parallel import
16.Application to change the distribution category of a product authorised through the centralised procedure
17.Application for a variation to a marketing authorisation dealt with under national or mutual recognition variation procedures.
18.Application for a variation to a marketing authorisation dealt with under worksharing procedures
19.Application for an extension dealt with under the decentralised procedure where the United Kingdom is the reference member State
20.Provision of information relating to the recognition of a United Kingdom marketing authorisation or an extension
21.Exception for a variation relating to animal testing
22.Application for the renewal of a national marketing authorisation
23.Application for the renewal of a marketing authorisation obtained through mutual recognition or the decentralised procedure
24.Registration of a homeopathic remedy
25.Renewal of a homeopathic remedy
26.Annual fees for marketing authorisations
27.Auditor’s certificate
PART 3 Fees payable by manufacturers
28.Application for a manufacturing authorisation
29.Application for a variation of a manufacturing authorisation
30.Application for an authorisation to manufacture an autogenous vaccine or a product for administration under the cascade
31.Annual fees
32.Site inspections – type of site
33.Inspection of a site where immunological veterinary medicinal products are manufactured
34.Inspection of a site where sterile veterinary medicinal products are manufactured
35.Inspection of a site where no immunological or sterile veterinary medicinal products are manufactured
36.Inspection of a site where veterinary medicinal products are assembled
37.Test sites
38.Animal blood bank or equine stem cell centre authorisations
PART 4 Fees relating to a wholesale dealer’s authorisation
39.Application for a wholesale dealer’s authorisation
40.Variation of a wholesale dealer’s authorisation
41.Annual fee for a wholesale dealer’s authorisation
42.Inspection of a wholesale dealer’s premises
PART 5 Fees relating to feedingstuffs
43.Fees for approvals and annual fees relating to feedingstuffs in Great Britain
44.Inspection fees relating to feedingstuffs in Great Britain
45.Fees payable in relation to feedingstuffs in Northern Ireland
46.Fees relating to premises for supply by suitably qualified persons
PART 6 General
47.Testing samples
48.Animal test certificates
49.Importation of a veterinary medicinal product for treatment under the cascade
50.Wholesale dealer’s import certificate
51.Specific batch control
52.Submission of control tests of an immunological product
53.Export certificates
54.Provision of advice
55.Appeals to the Veterinary Products Committee
56.Fee relating to an appointed person
57.Fees relating to a veterinary surgeon’s practice premises
58.Refund of fees relating to the Veterinary Products Committee or appointed persons
59.Fees relating to an improvement notice
60.Non-payment of fees
61.Waiver or reduction of fees
62.Reduction of fees when an application is withdrawn
PART 1Introduction
Interpretation
1. In this Schedule—
“national application” means an application for a marketing authorisation that does not involve another member State;
“pharmaceutical product” means any veterinary medicinal product other than an immunological product;
“simultaneous application” is an application in which, at the time an authorisation for a product is applied for, one or more additional applications are submitted for products that are identical to the first product except that—
in the case of an immunological product, they have a lesser number of antigens than the first product, but only contain antigens contained in the first product; and
in the case of a pharmaceutical product, they have different strengths of the active substance,
and, in the case of an application involving more than one member State, the additional applications do not include a member State that was not included in the first application.
Payment of fees
2. All fees under this Schedule are payable to the Secretary of State.
Time of payment
3. All fees are payable on invoice unless otherwise specified.
Multiple inspections
4. If a site, premises or establishment is inspected for more than one type of authorisation, approval or registration at the same time, the fee is the sum of —
(a)the highest fee payable; and
(b)50% of each of the other fees.
Expenses for inspections outside the United Kingdom
5. Whenever premises outside the United Kingdom are inspected, the travel and subsistence costs of the inspectors and interpreters’ fees are payable in addition to the inspection fee specified.
Translation
6. All translation costs are charged additionally.
PART 2Fees relating to marketing authorisations
Specified pharmaceutical applications
7. The following table sets out the fees relating to a pharmaceutical veterinary medicinal product for—
(a)a national application for a marketing authorisation that is—
(i)a full application under Part 1 of Schedule 1;
(ii)a bibliographic application; or
(iii)an application based on pharmacological equivalence;
(b)an application for a marketing authorisation using the decentralised procedure where the United Kingdom is a concerned member State;
(c)an application for the mutual recognition of a product authorised in another member State.
| Application | Full national application under Part 1 of Schedule 1 (£) | Bibliographic national application (£) | Pharmacologically equivalent national application | Decentralised application where the UK is a concerned member State or recognition of a product authorised in another member State (£) | ||
|---|---|---|---|---|---|---|
| Reference product authorised in UK (£) | Reference product not authorised in UK (£) | |||||
| Base Fee: | 13,530 | 12,115 | 7,195 | 9,220 | 6,515 | |
| Additional fee if any of the target species is a food-producing animal: | 3,905 | 3,585 | 2,155 | 2,760 | 1,415 | |
| Additional fee for each active ingredient not previously included in a veterinary medicinal product authorised in the United Kingdom— | ||||||
| food-producing animal: | 7,465 | 6,595 | 5,885 | 7,495 | 2,630 | |
| non-food-producing animal: | 6,525 | 5,855 | 5,590 | 7,155 | 2,295 | |
| Additional fee for each additional pack type: | 740 | 740 | 605 | 775 | 330 | |
| Additional fee for each additional active ingredient (food-producing animal): | 6,465 | 6,125 | 4,040 | 5,165 | 2,085 | |
| Additional fee for each additional active ingredient (non-food-producing animal): | 4,310 | 4,105 | 3,235 | 4,135 | 1,475 | |
| Additional fee if there is more than one target species, for each additional species (food-producing animal): | 3,970 | 3,565 | 2,425 | 3,100 | 1,280 Applies for a maximum of 2 additional species | |
| Additional fee if there is more than one target species, for each additional species (non- food-producing animal): | 2,495 | 2,090 | 1,550 | 1,980 | 805 Applies for a maximum of 2 additional species | |
| Additional fee for each additional recommended route of administration (food-producing animal): | 2,695 | 2,490 | 1,620 | 2,070 | 940 | |
| Additional fee for each additional recommended route of administration (non- food-producing animal): | 1,215 | 1,010 | 740 | 945 | 405 | |
| Simultaneous applications: fee for each additional product in the application: | 2,895 | 2,895 | 2,895 | 3,705 | 1,685 | |
Decentralised pharmaceutical application where the United Kingdom is the reference member State
8. The fee for a decentralised application for a pharmaceutical product where the United Kingdom is the reference member State is the same as for a national application as set out in the table in paragraph 7, with the addition of the fees in the following table.
Fees for decentralised pharmaceutical application where the United Kingdom is the reference member State
| Application | Additional fee for a pharmacologically equivalent product (£) | Additional fee otherwise (£) | |
|---|---|---|---|
| Food-producing animal: one member State: | 5,230 | 3,705 | |
| Non-food-producing animal: one member State: | 3,985 | 3,220 | |
| Each additional member State: | 530 | 530 | |
| Simultaneous application: fee for each additional product in the application: | |||
| one member State: | 6,670 | 6,670 | |
| each additional member State: | 120 | 120 | |
Application for a marketing authorisation for an immunological or biosimilar product
9.—(1) The fee for a national application for a marketing authorisation relating to an immunological or biosimilar product, a decentralised application where the United Kingdom is the concerned member State or the mutual recognition of a product authorised in another member State is in accordance with the following table.
(2) In this paragraph a biosimilar application means an application made in accordance with Article 13(4) of Directive 2001/82/EC and a biosimilar product means a product which is the subject of such an application.
Fees for specified immunological and biosimilar applications
| Application | National application for a marketing authorisation(£) | Decentralised application where the UK is a concerned member State or recognition of a product authorised in another member State (£) | |
|---|---|---|---|
| 1. Immunological or biosimilar product other than in paragraph 2 below: Base fee: | 11,775 | 5,785 | |
| The following fees are in addition to the base fee— | |||
| Additional fee for each active ingredient not previously included in a veterinary medicinal product authorised in the United Kingdom, and for each new combination of active ingredients: | 7,405 | 2,490 | |
| Additional fee for each adjuvant or preservative not previously included in a veterinary medicinal product authorised in the United Kingdom and for each new combination of adjuvants or preservatives: | 1,345 | 675 | |
| More than one antigenic component – fee for each additional component: | 1,350 | 405 | |
| More than one species – fee for each additional species: | 5,380 | 1,615 Applies for a maximum of 2 additional species | |
| More than one route of administration – fee for each additional route of administration: | 5,380 | 1,615 | |
| Simultaneous application - fee for each additional product in the application: | 2,895 | 1,685 | |
| 2. Immunological or product that is identical to a product already authorised in the United Kingdom but with a lesser number of antigens and that only contains antigens contained in that product: | 10,430 | 5,380 | |
Decentralised immunological application where the United Kingdom is the reference member State
10. The fee for a decentralised application for a marketing authorisation for an immunological product where the United Kingdom is the reference member State is the same as for a national application set out in the previous table, with the addition of the fees in the following table—
Fees for decentralised immunological application where the United Kingdom is the reference member State
| Application | Additional fee (£) | |
|---|---|---|
| One member State: | 3,470 | |
| Each additional member State: | 530 | |
| Simultaneous applications: fee for each additional product in the application: | ||
| one member State: | 6,670 | |
| each additional member State: | 120 | |
Applications for a marketing authorisation using data already assessed
11. The fees for applications for marketing authorisations using identical data submitted simultaneously or on the basis of information provided under Article 13(c) of Directive 2001/82/EC are in accordance with the following table.
Fees for a marketing authorisation using data already assessed
| Application | Fee (£)per authorisation | |
|---|---|---|
| Decentralised application where the United Kingdom is the reference member State— | ||
| one member State: | 4,165 | |
| each additional member State: | 530 | |
| Any other application: | 945 | |
Application for an exceptional marketing authorisation (pharmaceutical)
12. The fee for an application for an exceptional marketing authorisation for a pharmaceutical product is in accordance with the following table.
Fees for an exceptional marketing authorisation for a pharmaceutical product
| Application | Provisional (£) | Limited (£) | |
|---|---|---|---|
| Base Fee: | 12,015 | 6,765 | |
| The following fees are in addition to the base fee— | |||
| Additional fee if any of the target species is a food-producing animal: | 3,905 | 1,952 | |
| Additional fee for each active ingredient not previously included in a veterinary medicinal product authorised in the United Kingdom— | |||
| food-producing animal: | 5,850 | 3,732 | |
| non-food-producing animal: | 4,910 | 3,262 | |
| Additional fee for each additional pack type: | 710 | 370 | |
| Additional fee for each additional active ingredient (food-producing animal): | 5,955 | 3,232 | |
| Additional fee for each additional active ingredient (non-food-producing animal): | 3,800 | 2,155 | |
| Additional fee if there is more than one target species, for each additional species (food-producing animal): | 2,965 | 1,985 | |
| Additional fee if there is more than one target species, for each additional species (non-food-producing animal): | 1,485 | 1,247 | |
| Additional fee for each additional recommended route of administration (food-producing animal): | 2,185 | 1,347 | |
| Additional fee for each additional recommended route of administration (non-food-producing animal): | 710 | 608 | |
| Simultaneous applications— fee for each additional product in the application: | 2,895 | 1,447 | |
Fees for an application for an exceptional marketing authorisation (immunological)
13. The fee for an application for an exceptional marketing authorisation for an immunological product is in accordance with the following table.
Fees for an exceptional marketing authorisation for an immunological product
| Application | Provisional (£) | Limited (£) |
|---|---|---|
| Base fee: | 10,810 | 5,887 |
| The following fees are in addition to the base fee— | ||
| Additional fee for each active ingredient not previously included in a veterinary medicinal product authorised in the United Kingdom, and for each new combination of active ingredients: | 5,650 | 3,702 |
| Additional fee for each adjuvant or preservative not previously included in a veterinary medicinal product authorised in the United Kingdom and for each new combination of adjuvants or preservatives: | 1,350 | 672 |
| More than one antigenic component – fee for each additional component: | 1,190 | 675 |
| More than one species – fee for each additional species: | 4,060 | 2,690 |
| More than one route of administration – fee for each additional route of administration: | 4,060 | 2,690 |
| Simultaneous application - fee for each additional product in the application: | 2,895 | 1,447 |
Fee for the conversion from an exceptional to a full marketing authorisation
14. The fee for the conversion of an exceptional marketing authorisation to a full marketing authorisation is £3,000.
Application for a marketing authorisation relating to a parallel import
15. The fee for a marketing authorisation for a parallel import is in accordance with the following table.
Parallel imports
| Application | Fee (£) | |
|---|---|---|
| Application where the imported product has been authorised in accordance with the mutual recognition procedure or decentralised procedure, and the United Kingdom is included in these procedures— | ||
| import from one or more member States: | 1,755 | |
| Application to add an additional member State after the marketing authorisation has been granted – fee for each member State: | 455 | |
| Application where the imported product has not been authorised in accordance with the mutual recognition procedure or the decentralised procedure but where the imported product originates from the same manufacturing site as the product authorised in the United Kingdom to which the imported product is considered to be essentially similar: | 2,130 | |
| Any other application – fee for each member State from which the product is imported: | 4,710 | |
Application to change the distribution category of a product authorised through the centralised procedure
16. The fee to change the distribution category of a product authorised through the centralised procedure is £3,135.
Application for a variation to a marketing authorisation dealt with under national or mutual recognition variation procedures.
17.—(1) This paragraph applies in relation to an application for a variation to one or more marketing authorisations except where paragraph 18, 19 or 21 applies.
(2) The fees for the variations to which this paragraph applies are set out in the following table.
(3) Where applications are made at the same time seeking an identical change to the terms of more than one marketing authorisation, and those applications are based on identical data, fees are payable as for a grouped variation.
(4) References in this paragraph to a grouped variation being “led” by a particular type of variation indicate that the principal variation in that group is a variation of that type.
| Type of variation | National | UK is the reference member State | UK is a concerned member State | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Single variations; one change for each product | |||||||||
| Extension: | |||||||||
| Change of strength or potency or the addition of a new strength or potency: | 6,670 | - | 1,998 | ||||||
| Change of pharmaceutical form or the addition of a new pharmaceutical form: | 8,415 | - | 2,301 | ||||||
| Change of route of administration, or the addition of a new one, of— | - | ||||||||
| (i) an immunological product, or a pharmaceutical product for a food-producing animal: | 5,390 | - | 1,737 | ||||||
| (ii) a pharmaceutical product for a food-producing animal: | 7,135 | - | 2,058 | ||||||
| Change or addition of a food producing target species: | 9,620 | - | 2,547 | ||||||
| Change of active substance, including: | 8,415 | - | 2,301 | ||||||
| use of a different salt, ester, complex or derivative of the same therapeutic moiety: | |||||||||
| use of a different biologically active substance with a slightly different molecular structure: | |||||||||
| modification of the vector used to produce the antigen or the source material, including a new master cell bank from a different source: | |||||||||
| use of a new ligand or coupling mechanism for a radiopharmaceutical: | |||||||||
| change of the extraction solvent or change of the ratio of herbal drug to herbal drug preparation: | |||||||||
| Change of bioavailability: | 8,415 | - | 2,301 | ||||||
| Change of pharmacokinetics: | 8,415 | - | 2,301 | ||||||
| Simultaneous application: fee for each additional product in the application: | 2,895 | - | 1,011 | ||||||
| Type II: | 2,895 | 6,030 | 1,872 | ||||||
| Type IB: | 885 | 1,325 | 531 | ||||||
| Type IA: | 455 | 685 | 273 | ||||||
| Grouped variations | |||||||||
| Extension-led: | |||||||||
| The fee for an application for an extension-led grouped variation is the fee for that extension as specified above plus — | |||||||||
| (a) if there is one variation in addition to the extension, the fee for that variation as specified above; or | |||||||||
| (b) if there is more than one variation in addition to the extension, the fee that would be payable for a grouped variation of that type as specified below. | |||||||||
| Type II led: | |||||||||
| For the first nine changes: | 6,280 | 12,060 | 3,768 | ||||||
| For each subsequent group of up to ten changes: | 4,500 | 4,500 | 2,700 | ||||||
| Type IB led: | |||||||||
| For the first nine changes: | 1,770 | 2,650 | 1,062 | ||||||
| For each subsequent group of up to ten changes: | 4,500 | 4,500 | 2,700 | ||||||
| Type IA led: | |||||||||
| For the first nine changes: | 885 | 1,325 | 531 | ||||||
| For each subsequent group of up to ten changes: | 4,500 | 4,500 | 2,700 | ||||||
Application for a variation to a marketing authorisation dealt with under worksharing procedures
18.—(1) This paragraph applies in relation to an application for a variation to a marketing authorisation dealt with in accordance with worksharing procedures as set out in Article 20 of Commission Regulation (EC) No 1234/.
(2) The fee for a worksharing application, involving marketing authorisations obtained by a national procedure in the United Kingdom only, is the fee specified in the following table in the column headed “UK Only”.
(3) The fee for a worksharing application, involving marketing authorisations obtained through a national procedure in the United Kingdom and any other member State, is specified in the following table by reference to the United Kingdom’s role in the procedure, as “UK Reference Authority”, “UK Co-Reference Authority” or “Other”.
(4) The fee for a worksharing application, involving at least one marketing authorisation obtained through the mutual recognition or decentralised procedure, is specified in the following table by reference to the United Kingdom’s role in the procedure, as “UK Reference Authority”, “UK Co-Reference Authority” or “UK Concerned member State”.
(5) The fee for any kind of variation where the Agency co-ordinates worksharing is £455 for each marketing authorisation.
| Type of application | UK Only | Where the application involves nationally authorised products in more than one member State | Application involves mutually recognised products | ||||||
|---|---|---|---|---|---|---|---|---|---|
UK Only | UK Reference Authority | UK Co-Reference Authority | Other | UK Reference Authority | UK Co-Reference Authority | UK Concerned member State | |||
Worksharing applications The following fees apply for each change to each product: | |||||||||
| Type II | |||||||||
| For the first nine changes: | 6,240 | 12,060 | 7,485 | 12,060 | 13,265 | 6,745 | 3,372 | ||
| For each subsequent group of up to ten changes: | 4,500 | 4,500 | 4,500 | 4,500 | 4,500 | 4,500 | 2,700 | ||
| Type IB | |||||||||
| For the first nine changes: | 1,770 | 2,650 | 2,120 | 2,650 | 2,915 | 1,905 | 954 | ||
| For each subsequent group of up to ten changes: | 4,500 | 4,500 | 4,500 | 4,500 | 4,500 | 4,500 | 2,700 | ||
Application for an extension dealt with under the decentralised procedure where the United Kingdom is the reference member State
19. The fee for a decentralised application for an extension where the United Kingdom is the reference member State is the same as for a national application as set out in the table in paragraph 17, with the addition of the supplementary fees in the following table (save that, where the application is for the addition of more than one species, only one supplementary fee applies).
Decentralised application for an extension where the United Kingdom is the reference member State
| Application | Supplementary fee (£) | |
|---|---|---|
| Pharmaceutical product for a food-producing animal – one member State: | 3,705 | |
| Pharmaceutical product for a non-food-producing animal – one member State: | 3,220 | |
| Immunological product – one member State: | 3,460 | |
| Each additional member State: | 530 | |
| Simultaneous application: fee for each additional product in the application: | ||
| one member State: | 6,670 | |
| each additional member State: | 120 | |
Provision of information relating to the recognition of a United Kingdom marketing authorisation or an extension
20.—(1) Where an application is made for the Secretary of State to provide information to other member States to enable them to recognise a marketing authorisation already granted by the United Kingdom the following fees are payable.
(2) Those fees also apply where a marketing authorisation has been granted in more than one member State, the holder applies for an extension for that marketing authorisation and the United Kingdom acts as reference member State.
(3) Where a valid application to provide information to another member State is received within six months of the original grant of the marketing authorisation, or where the Secretary of State has already provided the information to a member State, and a further valid application is made to provide the information to an additional member State within six months of the date the last information was provided, the fees are—
| Type of application | Fee for a pharmacologically equivalent product(a) | Fee (other products) (£) | ||
|---|---|---|---|---|
(a) This fee is payable if the application for the marketing authorisation was on the basis that the product was pharmacologically equivalent to another veterinary medicinal product. | ||||
| Pharmaceutical product for a food-producing animal – one member State: | 3,940 | 2,440 | ||
| Pharmaceutical product for a non-food-producing animal ‑ one member State: | 2,645 | 1,895 | ||
| Immunological product – one member State: | 2,130 | 2,130 | ||
| Each additional member State: | 535 | 535 | ||
(4) Where the information to be provided relates to a product granted a marketing authorisation using identical data submitted simultaneously or on the basis of information provided under Article 13(c) of Directive 2001/82/EC the fees are—
| Application | Fee (£) | |
|---|---|---|
| Provision of information to— | ||
| one member State: | 4,165 | |
| each additional member State: | 530 | |
(5) In any other case the fees are—
| Type of application | Fee for a pharmacologically equivalent product (£)(a) | Fee (other products) (£) | |
|---|---|---|---|
(a) This fee is payable if the application for the marketing authorisation was on the basis that the product was pharmacologically equivalent to another veterinary medicinal product. | |||
| Pharmaceutical product for a food-producing animal – one member State: | 12,015 | 10,515 | |
| Pharmaceutical product for a non-food-producing animal – one member State: | 8,115 | 7,365 | |
| Immunological product – one member State: | 8,940 | 8,940 | |
| Each additional member State: | 535 | 535 | |
(6) In the case of simultaneous applications, the above fees are payable for each additional product in the application for one member State, with a fee of £115 for each additional product for each additional member State.
Exception for a variation relating to animal testing
21. If the only purpose of a variation is to remove animal testing or to reduce the numbers of animals used in testing, no fee is payable for the variation in the case of a national authorisation, and the United Kingdom element of the fee for the variation is not payable for an authorisation obtained through the mutual recognition procedure or the decentralised procedure.
Application for the renewal of a national marketing authorisation
22.—(1) The fee for an application for the renewal of a marketing authorisation is £1,360.
(2) The fee for the first reassessment of an exceptional marketing authorisation is £305, and the fee for each subsequent reassessment is £1,360.
Application for the renewal of a marketing authorisation obtained through mutual recognition or the decentralised procedure
23. The fee for an application for the renewal of a marketing authorisation obtained through mutual recognition or the decentralised procedure is —
(a)£1,835 if the United Kingdom is the reference member State; and
(b)£1,225 if the United Kingdom is a concerned member State.
Registration of a homeopathic remedy
24. The fee for an application for the registration of a homeopathic remedy is in accordance with the following table.
Fee for the registration of a homeopathic remedy
| Type of application | Fees(£) | ||
|---|---|---|---|
| If all stocks and the formulation have already been assessed by the Secretary of State— | |||
| not more than five stocks: | 160 | ||
| more than five stocks: | 375 | ||
| If either all the stocks have already been assessed by the Secretary of State but there is a new formulation, or if the formulation has already been assessed by the Secretary of State but one or more of the stocks have not been already assessed— | |||
| not more than five stocks: | 455 | ||
| more than five stocks: | 665 | ||
| If the formulation and at least one of the stocks has not already been assessed by the Secretary of State— | |||
| not more than five stocks: | 760 | ||
| more than five stocks: | 985 | ||
| If the product is already authorised for human use in the United Kingdom, or for human or veterinary use in the United Kingdom or in another member State— | |||
| not more than five stocks: | 160 | ||
| more than five stocks: | 375 | ||
Renewal of a homeopathic remedy
25. The fee for the renewal of a homeopathic remedy is £320.
Annual fees for marketing authorisations
26.—(1) Within 30 days of receiving a written demand from the Secretary of State, a holder of a marketing authorisation must provide the Secretary of State with a statement of turnover for the previous calendar year.
(2) The annual fee, rounded to the next £1, is—
where—
T is the annual turnover in the previous calendar year;
and n is the number of active marketing authorisations held at any time during the previous calendar year.
(3) In the case of an authorisation holder with a turnover relating to all marketing authorisations held of less than £230,000, the annual fee, rounded to the next £1 is—
where—
T is the annual turnover in the previous calendar year;
and n is the number of active marketing authorisations held at any time during the previous calendar year.
(4) In this paragraph—
“turnover” means the sales value at manufacturers’ prices of all authorised veterinary medicinal products sold or supplied in the United Kingdom;
“manufacturers’ prices” means the prices charged (excluding value added tax) for authorised products by manufacturers to wholesalers, except to the extent that—
the products are supplied by manufacturers direct to retailers, in which case it means the prices charged for the products by the manufacturers to the retailers reduced by such sum as, in the opinion of the Secretary of State, represents the difference between the prices paid by the retailers and those which could be expected to be charged by the manufacturers to wholesalers according to the practice prevailing during the period in question with regard to such products;
a marketing authorisation holder sells or supplies products that the marketing authorisation holder has neither manufactured nor obtained from the manufacturer, in which case it means the prices paid by the marketing authorisation holder for those products.
Auditor’s certificate
27.—(1) The Secretary of State may at any time require an audit certificate in support of a statement of turnover.
(2) If the holder of the marketing authorisation does not provide an audit certificate before the date stipulated in the demand, an additional fee is payable for that year of £11,300 plus an additional £2,245 in respect of each marketing authorisation held.
(3) If the Secretary of State is not satisfied that the audit certificate provides sufficient assurance that the figures fairly present the financial records of the company, the Secretary of State may require the marketing authorisation holder to produce a further certificate and specify what further assurances are needed; and if these are not provided by the required date, the additional fee specified in sub-paragraph (2) is payable.
(4) Nothing in this paragraph limits the powers of an inspector to examine financial records.
PART 3Fees payable by manufacturers
Application for a manufacturing authorisation
28. The fee for an application for a manufacturing authorisation for a veterinary medicinal product is—
(a)£3,040; or
(b)£530 if the authorisation only covers veterinary medicinal products manufactured under Schedule 6 (exemptions for small pet animals).
Application for a variation of a manufacturing authorisation
29. The fee for an application for the variation of a manufacturing authorisation is—
(a)£636 if the variation requires scientific or pharmaceutical assessment;
(b)£443 if the variation only involves a change of ownership;
(c)£210 if the authorisation only covers veterinary medicinal products manufactured under Schedule 6 (exemptions for small pet animals); and
(d)otherwise £350.
Application for an authorisation to manufacture an autogenous vaccine or a product for administration under the cascade
30.—(1) The fee for an application for a standard authorisation to manufacture an autogenous vaccine or a veterinary medicinal product for administration under the cascade is—
(a)£3,435 for a site in the United Kingdom;
(b)£3,270 for a site outside the United Kingdom.
(2) The fee for each inspection after a standard authorisation has been granted is (in each case) the same as the fee specified in paragraph (1).
(3) In the case of an application for an individual authorisation to manufacture a single batch of autogenous vaccine, or a single batch of veterinary medicinal product for administration under the cascade the fee is £1,635.
(4) The fee to vary an authorisation is £305 if no further inspection is required, and otherwise is the full application fee.
Annual fees
31.—(1) An annual fee of £550 is payable in respect of each manufacturing authorisation held (other than as specified in this paragraph).
(2) The annual fee for a manufacturing authorisation for an autogenous vaccine or a veterinary medicinal product for administration under the cascade is 0.67% of the turnover in the previous calendar year rounded to the next £1, with a minimum fee of £10.
(3) There is no annual fee for a manufacturing authorisation for a veterinary medicinal product manufactured in accordance with Schedule 6 for small pet animals.
(4) In this paragraph “turnover” means the sales value at manufacturers’ prices net of value added tax of all authorised veterinary medicinal products sold or supplied in the United Kingdom.
Site inspections – type of site
32. For the purposes of deciding the fee for a site inspection—
“super site” is a site at which 250 or more relevant persons are employed;
“major site” is a site at which 60 or more, but fewer than 250, relevant persons are employed;
“standard site” is a site at which 10 or more, but fewer than 60 relevant persons are employed;
“minor site” is a site at which fewer than 10 relevant persons are employed;
“relevant person” means a person employed on the premises and systems inspected.
Inspection of a site where immunological veterinary medicinal products are manufactured
33. The fees for the inspection of a site where immunological veterinary medicinal products are manufactured are in accordance with the following table.
Sites where immunological veterinary medicinal products are manufactured
| Type of site | Fee (£) | |
|---|---|---|
| United Kingdom site | Site outside United Kingdom | |
| Super site | 24,071 | 22,867 |
| Major site | 16,785 | 15,946 |
| Standard site | 6,661 | 6,327 |
| Minor site | 4,757 | 4,519 |
Inspection of a site where sterile veterinary medicinal products are manufactured
34. The following fees are payable for the inspection of a site where no immunological veterinary medicinal products are manufactured, but where sterile products are manufactured.
Sites where sterile veterinary medicinal products are manufactured
| Type of site | Fee (£) | |
|---|---|---|
| United Kingdom site | Site outside the United Kingdom | |
| Super site | 23,324 | 22,157 |
| Major site | 13,010 | 12,359 |
| Standard site | 8,244 | 7,832 |
| Minor site | 5,022 | 4,770 |
Inspection of a site where no immunological or sterile veterinary medicinal products are manufactured
35. The following fees are payable for the inspection of a site where only non-immunological and non-sterile veterinary medicinal products are manufactured—
Site where no immunological or sterile veterinary medicinal products are manufactured
| Type of site | Fee (£) | ||
|---|---|---|---|
| United Kingdom site | Site outside the United Kingdom | ||
| Super site | 14,180 | 13,471 | |
| Major site | 8,325 | 7,909 | |
| Standard site | 6,854 | 6,511 | |
| Minor site | 3,789 | 3,600 | |
| If the site is only involved in the manufacture of veterinary medicinal products authorised under Schedule 6 (exemptions for small pet animals— | |||
| Standard site | 5,055 | 4,802 | |
| Minor site | 2,728 | 2,592 | |
Inspection of a site where veterinary medicinal products are assembled
36. The following fees are payable for the inspection of a site where the only manufacturing process in relation to veterinary medicinal products is their assembly after the product has been put into its immediate container.
Site where medicinal products are assembled
| Type of site | Fee (£) | |
|---|---|---|
| United Kingdom site | Site outside the United Kingdom | |
| Super site | 11,025 | 10,474 |
| Major site | 5,949 | 5,652 |
| Standard site | 4,917 | 4,671 |
| Minor site | 2,035 | 1,933 |
Test sites
37. The fee for the inspection of a test site is £3,344, or £3,177 for a site outside the United Kingdom.
Animal blood bank or equine stem cell centre authorisations
38.—(1) The fee for an authorisation to operate a blood bank is—
(a)on a first inspection £3,113; and
(b)on each subsequent inspection—
(i)£3,113 for a site in the United Kingdom; and
(ii)£2,966 for a site outside the United Kingdom.
(2) The fee for an authorisation to operate an equine stem cell centre is £3,427, and £3,092 for each subsequent inspection.
(3) The fee for a variation to an authorisation to operate a blood-bank or equine stem cell centre is £320.
PART 4Fees relating to a wholesale dealer’s authorisation
Application for a wholesale dealer’s authorisation
39.—(1) The fee for an application for a wholesale dealer’s authorisation is—
(a)£1,745;
(b)£785 if the application is accompanied by an estimate that the first year’s turnover will be less than £35,000; or
(c)£785 if the authorisation only relates to products classified as AVM-GSL, homeopathic remedies, or products authorised under Schedule 6 (exemptions for small pet animals).
(2) An applicant who has paid a fee of £785 on the grounds of turnover must send a declaration of turnover for the first year of trading on the anniversary of the grant of the authorisation, and if the figure is more than £35,000 must pay the balance of £960 within 30 days.
(3) If the applicant paid £1,745 but the turnover for the first year of trading was lower than £35,000, if the applicant sends a declaration certifying the turnover, the Secretary of State must refund the excess.
(4) Nothing in this paragraph limits the powers of an inspector to examine financial records.
(5) In this paragraph “turnover” means the sales value net of value added tax of all veterinary medicinal products (whether or not authorised for use in the United Kingdom) sold by way of wholesale dealing by the holder in the United Kingdom.
Variation of a wholesale dealer’s authorisation
40. The fee for an application to vary a wholesale dealer’s authorisation is—
(a)£515 if the variation requires scientific or pharmaceutical assessment;
(b)£430 if the variation only involves a change of ownership; and
(c)otherwise £300.
Annual fee for a wholesale dealer’s authorisation
41.—(1) The annual fee for a wholesale dealer’s authorisation is—
(a)£483; or
(b)£315, if—
(i)the holder certifies when making the payment that the turnover during the previous year was less than £35,000; or
(ii)the authorisation only relates to products classified as AVM-GSL or homeopathic remedies;
(c)£215 if the authorisation only relates to products authorised under Schedule 6 (exemptions for small pet animals).
(2) In this paragraph “turnover” means the sales value net of value added tax of all veterinary medicinal products (whether or not authorised for use in the United Kingdom) sold by way of wholesale dealing by the holder in the United Kingdom.
Inspection of a wholesale dealer’s premises
42. The fee for the inspection of a wholesale dealer’s premises is—
(a)£3,058; or
(b)£1,442 if—
(i)the authorisation only relates to products classified as AVM-GSL or homeopathic remedies; or
(ii)the turnover relating to all veterinary medicinal products in the calendar year preceding the inspection was less than £35,000;
(c)£830 if the authorisation only relates to products authorised under Schedule 6 (exemptions for small pet animals).
PART 5Fees relating to feedingstuffs
Fees for approvals and annual fees relating to feedingstuffs in Great Britain
43.—(1) Subject to sub-paragraph (3) the fee for the application for approval of establishments manufacturing feedingstuffs and approval of distributors of feedingstuffs in Great Britain is £70.
(2) An annual fee of £70 is payable in respect of any such approval.
(3) No fee is payable under sub-paragraph (1) in respect of an establishment where specified feed additives are manufactured if a veterinary medicinal product intended to be incorporated into feedingstuffs is manufactured at that establishment in accordance with a manufacturing authorisation.
(4) Fees relating to feedingstuffs are payable with the application or on invoice for the subsequent annual fee.
(5) Where more than one manufacturing activity is carried out at one establishment only one fee (the highest) is payable.
Inspection fees relating to feedingstuffs in Great Britain
44. Fees for the inspection of establishments manufacturing or distributing feedingstuffs in Great Britain are in accordance with the following table.
Inspection fees
| Type of establishment inspected | Fee payable (£) | |
|---|---|---|
(1) No fee is payable for premises that already have a manufacturing authority relating to veterinary medicinal products for incorporating into feedingstuffs. | ||
| 1 | Establishment manufacturing a specified feed additive:(1) | 1,810 |
| 2 | Establishment manufacturing a premixture: | 1,090 |
| 3 | Establishment manufacturing feedingstuffs using specified feed additives and veterinary medicinal products directly at any concentration, or using premixtures or specified feed additive complementary feedingstuffs: | 1,090 |
| 4 | Establishment manufacturing feedingstuffs for placing on the market using a veterinary medicinal product or premixture where the concentration of veterinary medicinal product in the feedingstuffs is 2 kg per tonne or more: | 961 |
| 5 | Establishment manufacturing feedingstuffs using premixtures or specified feed additive complementary feedingstuffs containing specified feed additives when the feedingstuffs are to be placed on the market: | 405 |
| 6 | Establishment manufacturing feedingstuffs for the manufacturers own use using a veterinary medicinal product or premixture where the concentration of veterinary medicinal product in the feedingstuffs is 2 kg per tonne or more: | 320 |
| 7 | Establishment manufacturing feedingstuffs using premixtures containing specified feed additives when the feedingstuffs are to be used by the person manufacturing the feedingstuffs: | 240 |
| 8 | Establishment distributing specified feed additives, premixtures or feedingstuffs containing specified feed additives, or premixtures or complementary feedingstuffs containing veterinary medicinal products: | 227 |
Fees payable in relation to feedingstuffs in Northern Ireland
45.—(1) The annual fees payable for the approval of establishments manufacturing and distributing feedingstuffs in Northern Ireland are in accordance with the following table.
(2) Fees are payable with the application or, for the subsequent annual fee, on invoice.
(3) Where more than one manufacturing activity is carried out at one establishment only the highest fee is payable.
Approval fees
| Type of establishment | Fee payable (£) | |
|---|---|---|
(a) No fee is payable for establishments that already have a manufacturing authority relating to veterinary medicinal products for incorporating into feedingstuffs. | ||
| 1 | Establishment manufacturing a specified feed additive(a): | 545 |
| 2 | Establishment manufacturing a premixture: | 435 |
| 3 | Establishment manufacturing feedingstuffs using specified feed additives and veterinary medicinal products directly at any concentration, or using premixtures or specified feed additive complementary feedingstuffs: | 435 |
| 4 | Establishment manufacturing feedingstuffs for placing on the market using a veterinary medicinal product or premixture where the concentration of veterinary medicinal product in the feedingstuffs is 2 kg per tonne or more: | 320 |
| 5 | Establishment manufacturing feedingstuffs using premixtures or specified feed additive complementary feedingstuffs containing specified feed additives when the feedingstuffs are to be placed on the market: | 170 |
| 6 | Establishment manufacturing feedingstuffs for the manufacturers own use using a veterinary medicinal product or premixture where the concentration of veterinary medicinal product in the feedingstuffs is 2 kg per tonne or more: | 131 |
| 7 | Establishment manufacturing feedingstuffs using premixtures containing specified feed additives when the feedingstuffs are to be used by the person manufacturing the feedingstuffs: | 110 |
| 8 | Establishment distributing specified feed additives, premixtures or feedingstuffs containing specified feed additives, or premixtures or feedingstuffs containing veterinary medicinal products: | 70 |
Fees relating to premises for supply by suitably qualified persons
46.—(1) The fee to approve of premises for the retail supply of veterinary medicinal products by suitably qualified persons is—
(a)£265; or
(b)if the premises are only authorised to supply veterinary medicinal products for the treatment of—
(i)horses (or horses and companion animals) £145; or
(ii)companion animals £110.
(2) The subsequent annual fee is—
(a)£185; or
(b)if the premises are only authorised to supply veterinary medicinal products for the treatment of—
(i)horses (or horses and companion animals) £95; or
(ii)companion animals £70.
PART 6General
Testing samples
47. The fee for testing a sample required to be submitted by the Secretary of State is the full economic cost of the test.
Animal test certificates
48.—(1) The fee for an animal test certificate is £345 in the case of—
(a)an immunological veterinary medicinal product that has been authorised in another member State for the species on which the proposed test will be conducted;
(b)a pharmaceutical veterinary medicinal product that has been authorised in another member State for use with a food-producing species on which the proposed test will be conducted where the same or similar dosage regime and method of administration is to be used in the medicinal test as is authorised; or
(c)a pharmaceutical veterinary medicinal product authorised in another member State for human or animal use where the test is to be conducted on companion animals only.
(2) The fee for an animal test certificate to administer medicinal products in a small scale trial to test them for clinical safety or efficacy is £30.
(3) In any other case the fee is £815.
(4) The fee for an application for a variation of the certificate is £265 for each change.
(5) The fee for an application to renew a certificate is £130.
(6) The Secretary of State may waive the fee if satisfied that the application is in relation to developing a veterinary medicinal product for a limited market (for example, for a minor species, a minor use, or for a disease with restricted regional distribution).
Importation of a veterinary medicinal product for treatment under the cascade
49.—(1) The fee for a certificate to import (if necessary) and be in possession of and administer a veterinary medicinal product under the cascade is—
(a)£15 if the veterinary medicinal product is authorised in another member State;
(b)£30 if the veterinary medicinal product is authorised in a third country.
(2) The fee is payable in respect of each animal treated, but in the case of administration to and treatment of a discrete group of animals, the Secretary of State may notify the applicant in writing that a fee for only one animal is payable.
(3) There is no fee if the application is made using the website of the Veterinary Medicines Directorate.
Wholesale dealer’s import certificate
50.—(1) The fee payable by the holder of a wholesale dealer’s authorisation for a certificate to import and store a veterinary medicinal product not authorised in the United Kingdom to enable it to be supplied for administration under Schedule 4 is £1,320.
(2) The fee is only payable if, in the twelve month period immediately before the application, the applicant has supplied the veterinary medicinal product to which the certificate relates in accordance with at least 100 certificates.
Specific batch control
51. The fee for an authorisation to release a veterinary medicinal product, under specific batch control is—
(a)£560; or
(b)£100 for each batch if a number of specific batch control applications are made at the same time and all the batches are affected by the same issue.
Submission of control tests of an immunological product
52. The fee for the submission of the results of tests carried out on a batch of immunological products other than autogenous vaccines prior to release is £80.
Export certificates
53. The fee for an application for an export certificate is £30, and £15 for each certified copy.
Provision of advice
54. The fee for an application for written advice from the Secretary of State as to whether or not a product requires a marketing authorisation is £885.
Appeals to the Veterinary Products Committee
55. The fee for an appeal to the Veterinary Products Committee is £1,500.
Fee relating to an appointed person
56. The appellant is liable for the full economic cost of a referral to an appointed person subject to a maximum of £5,000.
Fees relating to a veterinary surgeon’s practice premises
57.—(1) The fee for the inspection of a veterinary surgeon’s practice premises is £350.
(2) The initial registration and annual fee for the registration of veterinary practice premises with the Royal College of Veterinary Surgeons to supply veterinary medicinal products is £34.
(3) Notwithstanding paragraph 2 of this Schedule, this is payable to the Royal College of Veterinary Surgeons.
Refund of fees relating to the Veterinary Products Committee or appointed persons
58. The Secretary of State must refund the fee payable in relation to an appeal to the Veterinary Products Committee or to an appointed person if, as a result of the appeal, the Secretary of State changes the decision that was the subject of the appeal.
Fees relating to an improvement notice
59. If an improvement notice is served under these Regulations, the fee for any subsequent inspection necessary as a result of the notice is the full economic cost of the inspection, payable by the person on whom the notice was served.
Non-payment of fees
60. Where any fee (other than any fee relating to a manufacturing authorisation or wholesale dealer’s authorisation) is not paid, the Secretary of State may, after giving one month’s written warning, suspend the processing of any application from the person who has not paid the fee.
Waiver or reduction of fees
61.—(1) If the Secretary of State is satisfied that for reasons of human or animal health or the protection of the environment it is desirable that a product should be authorised for veterinary use or that an authorised product should remain on the market the Secretary of State may waive or reduce any fees payable under these Regulations.
(2) An applicant or the holder of a marketing authorisation must provide full written justification for any waiver or reduction.
Reduction of fees when an application is withdrawn
62.—(1) Where an application for a marketing authorisation, or any variation referred to in paragraph 17 or 18 as a Type II variation, an extension, an extension-led grouped variation or a Type II led grouped variation is withdrawn before determination, the fee is reduced in accordance with this paragraph.
(2) If no assessment (veterinary, scientific or pharmaceutical) has begun, the reduction is 90%.
(3) If assessment has begun but the Secretary of State has not yet requested further data, the reduction is 50%.
(4) If the Secretary of State has requested further information but it has not yet been provided, the reduction is 25%.
(5) If the further information requested has been supplied but has not yet been fully assessed or the application has not been referred to the Veterinary Products Committee, the reduction is 10%
(6) Once the further information has been fully assessed, or the application has been referred to the Veterinary Products Committee, there is no reduction.
Options/Help
Print Options
PrintThe Whole Instrument
PrintThis Schedule only
You have chosen to open The Whole Instrument
The Whole Instrument you have selected contains over 200 provisions and might take some time to download. You may also experience some issues with your browser, such as an alert box that a script is taking a long time to run.
Would you like to continue?
You have chosen to open The Whole Instrument as a PDF
The Whole Instrument you have selected contains over 200 provisions and might take some time to download.
Would you like to continue?
You have chosen to open the Whole Instrument
The Whole Instrument you have selected contains over 200 provisions and might take some time to download. You may also experience some issues with your browser, such as an alert box that a script is taking a long time to run.
Would you like to continue?
You have chosen to open Schedules only
The Schedules you have selected contains over 200 provisions and might take some time to download. You may also experience some issues with your browser, such as an alert box that a script is taking a long time to run.
Would you like to continue?
Legislation is available in different versions:
Latest Available (revised):The latest available updated version of the legislation incorporating changes made by subsequent legislation and applied by our editorial team. Changes we have not yet applied to the text, can be found in the ‘Changes to Legislation’ area.
Original (As Enacted or Made): The original version of the legislation as it stood when it was enacted or made. No changes have been applied to the text.
Explanatory Memorandum
Explanatory Memorandum sets out a brief statement of the purpose of a Statutory Instrument and provides information about its policy objective and policy implications. They aim to make the Statutory Instrument accessible to readers who are not legally qualified and accompany any Statutory Instrument or Draft Statutory Instrument laid before Parliament from June 2004 onwards.
More Resources
Access essential accompanying documents and information for this legislation item from this tab. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as enacted version that was used for the print copy
- lists of changes made by and/or affecting this legislation item
- confers power and blanket amendment details
- all formats of all associated documents
- correction slips
- links to related legislation and further information resources
Impact Assessments
Impact Assessments generally accompany all UK Government interventions of a regulatory nature that affect the private sector, civil society organisations and public services. They apply regardless of whether the regulation originates from a domestic or international source and can accompany primary (Acts etc) and secondary legislation (SIs). An Impact Assessment allows those with an interest in the policy area to understand:
- Why the government is proposing to intervene;
- The main options the government is considering, and which one is preferred;
- How and to what extent new policies may impact on them; and,
- The estimated costs and benefits of proposed measures.
More Resources
Use this menu to access essential accompanying documents and information for this legislation item. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as made version that was used for the print copy
- correction slips
Click 'View More' or select 'More Resources' tab for additional information including:
- lists of changes made by and/or affecting this legislation item
- confers power and blanket amendment details
- all formats of all associated documents
- links to related legislation and further information resources