- Y Diweddaraf sydd Ar Gael (Diwygiedig)
- Gwreiddiol (Fel y’i mabwysiadwyd gan yr UE)
Directive 98/79/EC of the European Parliament and of the CouncilDangos y teitl llawn
Directive 98/79/EC of the European Parliament and of the Council of 27 October 1998 on in vitro diagnostic medical devices
You are here:
- Cyfarwyddebau yn deillio o’r UE
- 1998 No. 79
- Annexes only
- Dangos Graddfa Ddaearyddol(e.e. Lloegr, Cymru, Yr Alban aca Gogledd Iwerddon)
- Dangos Llinell Amser Newidiadau
Rhagor o Adnoddau
PDF o Fersiynau Diwygiedig
- ddiwygiedig 11/01/20120.39 MB
- ddiwygiedig 07/08/20090.19 MB
- ddiwygiedig 20/11/20030.18 MB
- ddiwygiedig 07/12/19980.18 MB
Mae hon yn eitem o ddeddfwriaeth sy’n deillio o’r UE
Mae unrhyw newidiadau sydd wedi cael eu gwneud yn barod gan y tîm yn ymddangos yn y cynnwys a chyfeirir atynt gydag anodiadau.Ar ôl y diwrnod ymadael bydd tair fersiwn o’r ddeddfwriaeth yma i’w gwirio at ddibenion gwahanol. Y fersiwn legislation.gov.uk yw’r fersiwn sy’n weithredol yn y Deyrnas Unedig. Y Fersiwn UE sydd ar EUR-lex ar hyn o bryd yw’r fersiwn sy’n weithredol yn yr UE h.y. efallai y bydd arnoch angen y fersiwn hon os byddwch yn gweithredu busnes yn yr UE.
Y fersiwn yn yr archif ar y we yw’r fersiwn swyddogol o’r ddeddfwriaeth fel yr oedd ar y diwrnod ymadael cyn cael ei chyhoeddi ar legislation.gov.uk ac unrhyw newidiadau ac effeithiau a weithredwyd yn y Deyrnas Unedig wedyn. Mae’r archif ar y we hefyd yn cynnwys cyfraith achos a ffurfiau mewn ieithoedd eraill o EUR-Lex.
Changes over time for: Directive 98/79/EC of the European Parliament and of the Council (Annexes only)
Status:
EU Directives are being published on this site to aid cross referencing from UK legislation. After IP completion day (31 December 2020 11pm) no further amendments will be applied to this version.
ANNEX IU.K.ESSENTIAL REQUIREMENTS
A.GENERAL REQUIREMENTSU.K.
1.The devices must be designed and manufactured in such a way that, when used under the conditions and for the purposes intended, they will not compromise, directly or indirectly, the clinical condition or the safety of the patients, the safety or health of users or, where applicable, other persons, or the safety of property. Any risks which may be associated with their use must be acceptable when weighed against the benefits to the patient and be compatible with a high level of protection of health and safety.U.K.
2.The solutions adopted by the manufacturer for the design and construction of the devices must conform to safety principles, taking account of the generally acknowledged state of the art.U.K.
In selecting the most appropriate solutions, the manufacturer must apply the following principles in the following order:
eliminate or reduce risks as far as possible (inherently safe design and construction),
where appropriate take adequate protection measures in relation to risks that cannot be eliminated,
inform users of the residual risks due to any shortcomings of the protection measures adopted.
3.The devices must be designed and manufactured in such a way that they are suitable for the purposes referred to in Article 1(2)(b), as specified by the manufacturer, taking account of the generally acknowledged state of the art. They must achieve the performances, in particular, where appropriate, in terms of analytical sensitivity, diagnostic sensitivity, analytical specificity, diagnostic specificity, accuracy, repeatability, reproducibility, including control of known relevant interference, and limits of detection, stated by the manufacturer.U.K.
The traceability of values assigned to calibrators and/or control materials must be assured through available reference measurement procedures and/or available reference materials of a higher order.
4.The characteristics and performances referred to in sections 1 and 3 must not be adversely affected to such a degree that the health or the safety of the patient or the user and, where applicable, of other persons, are compromised during the lifetime of the device as indicated by the manufacturer, when the device is subjected to the stresses which can occur during normal conditions of use. When no lifetime is stated, the same applies for the lifetime reasonably to be expected of a device of that kind, having regard to the intended purpose and the anticipated use of the device.U.K.
5.The devices must be designed, manufactured and packed in such a way that their characteristics and performances during their intended use will not be adversely affected under storage and transport conditions (temperature, humidity, etc.) taking account of the instructions and information provided by the manufacturer.U.K.
B.DESIGN AND MANUFACTURING REQUIREMENTSU.K.
1.Chemical and physical propertiesU.K.
1.1.The devices must be designed and manufactured in such a way as to achieve the characteristics and performances referred to in section A on the ‘General requirements’. Particular attention must be paid to the possibility of impairment of analytical performance due to incompatibilitybetween the materials used and the specimens (such as biological tissues, cells, body fluids and micro-organisms) intended to be used with the device, taking account of its intended purpose.U.K.
1.2.The devices must be designed, manufactured and packed in such a way as to reduce as far as possible the risk posed by product leakage, contaminants and residues to the persons involved in the transport, storage and use of the devices, taking account of the intended purpose of the products.U.K.
2.Infection and microbial contaminationU.K.
2.1.The devices and their manufacturing processes must be designed in such a way as to eliminate or reduce as far as possible the risk of infection to the user or other persons. The design must allow easy handling and, where necessary, reduce as far as possible contamination of, and leakage from, the device during use and, in the case of specimen receptacles, the risk of contamination of the specimen. The manufacturing processes must be appropriate for these purposes.U.K.
2.2Where a device incorporates biological substances, the risks of infection must be reduced as far as possible by selecting appropriate donors and appropriate substances and by using appropriate, validated inactivation, conservation, test and control procedures.U.K.
2.3.Devices labelled either as ‘STERILE’ or as having a special microbiological state must be designed, manufactured and packed in an appropriate pack, according to procedures suitable for ensuring that they remain in the appropriate microbiological state indicated on the label when placed on the market, under the storage and transport conditions specified by the manufacturer, until the protective packaging is damaged or opened.U.K.
2.4.Devices labelled either as ‘STERILE’ or as having a special microbiological state must have been processed by an appropriate, validated method.U.K.
2.5.Packaging systems for devices other than those referred to in section 2.3 must keep the product without deterioration at the level of cleanliness indicated by the manufacturer and, if the devices are to be sterilised prior to use, reduce as far as possible the risk of microbial contamination.U.K.
Steps must be taken to reduce as far as possible microbial contamination during selection and handling of raw materials, manufacture, storage and distribution where the performance of the device can be adversely affected by such contamination.
2.6.Devices intended to be sterilised must be manufactured in appropriately controlled (e.g. environmental) conditions.U.K.
2.7.Packaging systems for non-sterile devices must keep the product without deterioration at the level of cleanliness stipulated and, if the devices are to be sterilised prior to use, minimise the risk of microbial contamination; the packaging system must be suitable taking account of the method of sterilisation indicated by the manufacturer.U.K.
3.Manufacturing and environmental propertiesU.K.
3.1.If the device is intended for use in combination with other devices or equipment, the whole combination, including the connection system, must be safe and must not impair the specified performances of the devices. Any restrictions on use must be indicated on the label and/or in the instructions for use.U.K.
3.2.Devices must be designed and manufactured in such a way as to reduce as far as possible the risks linked to their use in conjunction with materials, substances and gases with which they may come into contact during normal conditions of use.U.K.
3.3.Devices must be designed and manufactured in such a way as to remove or reduce as far as possible:U.K.
the risk of injury linked to their physical features (in particular aspects of volume × pressure, dimension and, where appropriate, ergonomic features),
risks linked to reasonably foreseeable external influences, such as magnetic fields, external electrical effects, electrostatic discharge, pressure, humidity, temperature or variations in pressure or acceleration or accidental penetration of substances into the device.
Devices must be designed and manufactured in such a way as to provide an adequate level of intrinsic immunity of electromagnetic disturbance to enable them to operate as intended.
3.4.Devices must be designed and manufactured in such a way as to reduce as far as possible the risks of fire or explosion during normal use and in single fault condition. Particular attention must be paid to devices whose intended use includes exposure to or use in association with flammable substances or substances which could cause combustion.U.K.
3.5.Devices must be designed and manufactured in such a way as to facilitate the management of safe waste disposal.U.K.
3.6.The measuring, monitoring or display scale (including colour change and other visual indicators) must be designed and manufactured in line with ergonomic principles, taking account of the intended purpose of the device.U.K.
4.Devices which are instruments or apparatus with a measuring functionU.K.
4.1.Devices which are instruments or apparatus having a primary analytical measuring function must be designed and manufactured in such a way as to provide adequate stability and accuracy of measurement within appropriate accuracy limits, taking into account the intended purpose of the device and of available and appropriate reference measurement procedures and materials. The accuracy limits have to be specified by the manufacturer.U.K.
4.2.When values are expressed numerically, they must be given in legal units conforming to the provisions of Council Directive 80/181/EEC of 20 December 1979 on the approximation of the laws of the Member States relating to units of measurement(1).U.K.
5.Protection against radiationU.K.
5.1.Devices shall be designed, manufactured and packaged in such a way that exposure of users and other persons to the emitted radiation is minimised.U.K.
5.2.When devices are intended to emit potentially hazardous, visible and/or invisible radiation, they must as far as possible be:U.K.
designed and manufactured in such a way as to ensure that the characteristics and the quantity of radiation emitted can be controlled and/or adjusted,
fitted with visual displays and/or audible warnings of such emissions.
5.3.The operating instructions for devices emitting radiation must give detailed information as to the nature of the emitted radiation, means of protecting the user, and on ways of avoiding misuse and of eliminating the risks inherent in installation.U.K.
6.Requirements for medical devices connected to or equipped with an energy sourceU.K.
6.1.Devices incorporating electronic programmable systems, including software, must be designed to ensure the repeatability, reliability and performance of these systems according to the intended use.U.K.
6.2.Devices must be designed and manufactured in such a way as to minimise the risks of creating electromagnetic perturbation which could impair the operation of other devices or equipment in the usual environment.U.K.
6.3.Devices must be designed and manufactured in such a way as to avoid, as far as possible, the risk of accidental electric shocks during normal use and in single fault condition, provided the devices are installed and maintained correctly.U.K.
6.4.Protection against mechanical and thermal risksU.K.
6.4.1.Devices must be designed and manufactured in such a way as to protect the user against mechanical risks. Devices must be sufficiently stable under the foreseen operating conditions. They must be suitable to withstand stresses inherent in the foreseen working environment, and to retain this resistance during the expected life of the devices, subject to any inspection and maintenance requirements as indicated by the manufacturer.U.K.
Where there are risks due to the presence of moving parts, risks due to break-up or detachment, or leakage of substances, then appropriate protection means must be incorporated.
Any guards or other means included with the device to provide protection, in particular against moving parts, must be secure and must not interfere with access for the normal operation of the device, or restrict routine maintenance of the device as intended by the manufacturer.
6.4.2.Devices must be designed and manufactured in such a way as to reduce to the lowest possible level the risks arising from vibration generated by the devices, taking account of technical progress and of the means available for limiting vibrations, particularly at source, unless the vibrations are part of the specified performance.U.K.
6.4.3.Devices must be designed and manufactured in such a way as to reduce as far as possible the risks arising from the noise emitted, taking account of technical progress and of the means available to reduce noise, particularly at source, unless the noise emitted is part of the specified performance.U.K.
6.4.4.Terminals and connectors to electricity, gas or hydraulic and pneumatic energy supplies which the user has to handle must be designed and manufactured in such a way as to minimise all possible risks.U.K.
6.4.5.Accessible parts of the devices (excluding the parts of areas intended to supply heat or reach given temperatures) and their surroundings must not attain potentially dangerous temperatures under normal use.U.K.
7.Requirements for devices for self-testingU.K.
Devices for self-testing must be designed and manufactured in such a way that they perform appropriately for their intended purpose taking into account the skills and the means available to users and the influence resulting from variation that can reasonably be anticipated in users' technique and environment. The information and instructions provided by the manufacturer should be easily understood and applied by the user.
7.1.Devices for self-testing must be designed and manufactured in such a way as to:U.K.
ensure that the device is easy to use by the intended lay user at all stages of the procedure, and
reduce as far as practicable the risk of user error in the handling of the device and in the interpretation of the results.
7.2.Devices for self-testing must, where reasonably possible, include user control, i.e. a procedure by which the user can verify that, at the time of use, the product will perform as intended.U.K.
8.Information supplied by the manufacturerU.K.
8.1.Each device must be accompanied by the information needed to use it safely and properly, taking account of the training and knowledge of the potential users, and to identify the manufacturer.U.K.
This information comprises the data on the label and in the instructions for use.
As far as practicable and appropriate, the information needed to use the device safely and properly must be set out on the device itself and/or, where appropriate, on the sales packaging. If individual full labelling of each unit is not practicable, the information must be set out on the packaging and/or in the instructions for use supplied with one or more devices.
Instructions for use must accompany or be included in the packaging of one or more devices.
In duly justified and exceptional cases no such instructions for use are needed for a device if it can be used properly and safely without them.
[X1The decision whether to translate the instructions for use and the label into one or more languages of the European Union shall be left to the Member States, except that, for devices for self-testing, the instructions for use and the label must include a translation into the official language(s) of the Member State in which the device for self-testing reaches its final user.]
Editorial Information
8.2.Where appropriate, the information to be supplied should take the form of symbols. Any symbol and identification colour used must conform to the harmonised standards. In areas for which no standards exist, the symbols and colour used must be described in the documentation supplied with the device.U.K.
8.3.In the case of devices containing or a preparation which may be considered as being dangerous, taking account of the nature and quantity of its constituents and the form under which they are present, relevant danger symbols and labelling requirements of Directive 67/548/EEC(2) and Directive 88/379/EEC(3) shall apply. Where there is insufficient space to put all the information on the device itself or on its label, the relevant danger symbols shall be put on the label and the other information required by those Directives shall be given in the instructions for use.U.K.
The provisions of the aforementioned Directives on the safety data sheet shall apply, unless all relevant information as appropriate is already made available by the instructions for use.
8.4.The label must bear the following particulars which may take the form of symbols as appropriate:U.K.
the name or trade name and address of the manufacturer. For devices imported into the Community with a view to their distribution in the Community, the label, the outer packaging, or the instructions for use shall contain in addition the name and address of the authorised representative of the manufacturer;
the details strictly necessary for the user to uniquely identify the device and the contents of the packaging;
where appropriate, the word ‘STERILE’ or a statement indicating any special microbiological state or state of cleanliness;
the batch code, preceded by the word ‘LOT’, or the serial number;
if necessary, an indication of the date by which the device or part of it should be used, in safety, without degradation of performance, expressed as the year, the month and, where relevant, the day, in that order;
in case of devices for performance evaluation, the words ‘for performance evaluation only’;
where appropriate, a statement indicating the in vitro use of the device;
any particular storage and/or handling conditions;
where applicable, any particular operating instructions;
appropriate warnings and/or precautions to take;
if the device is intended for self-testing, that fact must be clearly stated.
8.5.If the intended purpose of the device is not obvious to the user, the manufacturer must clearly state the intended purpose in the instructions for use and, if appropriate, on the label.U.K.
8.6.Wherever reasonable and practicable, the devices and separate components must be identified, where appropriate in terms of batches, to allow all appropriate action to detect any potential risk posed by the devices and detachable components.U.K.
8.7.Where appropriate, the instructions for use must contain the following particulars:U.K.
the details referred to in section 8.4 with the exception of points (d) and (e);
composition of the reagent product by nature and amount or concentration of the active ingredient(s) of the reagent(s) or kit as well as a statement, where appropriate, that the device contains other ingredients which might influence the measurement;
the storage conditions and shelf life following the first opening of the primary container, together with the storage conditions and stability of working reagents;
the performances referred to in section 3 of part A;
an indication of any special equipment required including information necessary for the identification of that special equipment for proper use;
the type of specimen to be used, any special conditions of collection, pre-treatment and, if necessary, storage conditions and instructions for the preparation of the patient;
a detailed description of the procedure to be followed in using the device;
the measurement procedure to be followed with the device including as appropriate:
the principle of the method,
the specific analytical performance characteristics (e.g. sensitivity, specificity, accuracy, repeatability, reproducibility, limits of detection and measurement range, including information needed for the control of known relevant interferences), limitations of the method and information about the use of available reference measurement procedures and materials by the user,
the details of any further procedure or handling needed before the device can be used (for example, reconstitution, incubation, dilution, instrument checks, etc.),
the indication whether any particular training is required;
the mathematical approach upon which the calculation of the analytical result is made;
measures to be taken in the event of changes in the analytical performance of the device;
information appropriate to users on:
internal quality control including specific validation procedures,
the traceability of the calibration of the device;
the reference intervals for the quantities being determined, including a description of the appropriate reference population;
if the device must be used in combination with or installed with or connected to other medical devices or equipment in order to operate as required for its intended purpose, sufficient details of its characteristics to identify the correct devices or equipment to use in order to obtain a safe and proper combination;
all the information needed to verify whether the device is properly installed and can operate correctly and safely, plus details of the nature and frequency of the maintenance and calibration needed to ensure that the device operates properly and safely; information about safe waste disposal;
details of any further treatment or handling needed before the device can be used (for example, sterilisation, final assembly, etc.);
the necessary instructions in the event of damage to the protective packaging and details of appropriate methods of resterilisation or decontamination;
if the device is reusable, information on the appropriate processes to allow reuse, including cleaning, disinfection, packaging and resterilisation or decontamination, and any restriction on the number of reuses;
precautions to be taken as regards exposure, in reasonably foreseeable environmental conditions, to magnetic fields, external electrical influences, electrostatic discharge, pressure or variations in pressure, acceleration, thermal ignition sources, etc.;
precautions to be taken against any special, unusual risks related to the use or disposal of the device including special protective measures; where the device includes substances of human or animal origin, attention must be drawn to their potential infectious nature;
specifications for devices for self-testing:
the results need to be expressed and presented in a way that is readily understood by a lay person; information needs to be provided with advice to the user on action to be taken (in case of positive, negative or indeterminate result) and on the possibility of false positive or false negative result,
specific particulars may be omitted provided that the other information supplied by the manufacturer is sufficient to enable the user to use the device and to understand the result(s) produced by the device,
the information provided must include a statement clearly directing that the user should not take any decision of medical relevance without first consulting his or her medical practitioner,
the information must also specify that when the device for self-testing is used for the monitoring of an existing disease, the patient should only adapt the treatment if he has received the appropriate training to do so;
date of issue or latest revision of the instructions for use.
ANNEX IIU.K.LIST OF DEVICES REFERRED TO IN ARTICLE 9(2) AND (3)
List A
Reagents and reagent products, including related calibrators and control materials, for determining the following blood groups: ABO system, rhesus (C, c, D, E, e) anti-Kell,
reagents and reagent products, including related calibrators and control materials, for the detection, confirmation and quantification in human specimens of markers of HIV infection (HIV 1 and 2), HTLV I and II, and hepatitis B, C and D[F1,]
[F2variant Creutzfeldt-Jakob disease (vCJD) assays for blood screening, diagnosis and confirmation.]
Textual Amendments
List B
Reagents and reagent products, including related calibrators and control materials, for determining the following blood groups: anti-Duffy and anti-Kidd,
reagents and reagent products, including related calibrators and control materials, for determining irregular anti-erythrocytic antibodies,
reagents and reagent products, including related calibrators and control materials, for the detection and quantification in human samples of the following congenital infections: rubella, toxoplasmosis,
reagents and reagent products, including related calibrators and control materials, for diagnosing the following hereditary disease: phenylketonuria,
reagents and reagent products, including related calibrators and control materials, for determining the following human infections: cytomegalovirus, chlamydia,
reagents and reagent products, including related calibrators and control materials, for determining the following HLA tissue groups: DR, A, B,
reagents and reagent products, including related calibrators and control materials, for determining the following tumoral marker: PSA,
reagents and reagent products, including related calibrators, control materials and software, designed specifically for evaluating the risk of trisomy 21,
the following device for self-diagnosis, including its related calibrators and control materials: device for the measurement of blood sugar.
ANNEX IIIU.K.EC DECLARATION OF CONFORMITY
1.The EC declaration of conformity is the procedure whereby the manufacturer or his authorised representative who fulfils the obligations imposed by section 2 to 5 and additionally, in the case of devices for self-testing, the obligations imposed by section 6, ensures and declares that the products concerned meet the provisions of this Directive which apply to them. The manufacturer must affix the CE marking in accordance with Article 16.U.K.
2.The manufacturer must prepare the technical documentation described in section 3 and ensure that the manufacturing process follows the principles of quality assurance as set out in section 4.U.K.
3.The technical documentation must allow assessment of the conformity of the product with the requirements of the Directive. It must include in particular:U.K.
a general description of the product, including any variants planned,
the documentation of the quality system,
design information, including the determination of the characteristics of the basic materials, characteristics and limitation of the performance of the devices, methods of manufacture and, in the case of instruments, design drawings, diagrams of components, sub-assemblies, circuits, etc.,
in the case of devices containing tissues of human origin or substances derived from such tissue, information on the origin of such material and on the conditions in which it was collected,
the descriptions and explanations necessary to understand the abovementioned characteristics, drawings and diagrams and the operation of the product,
the results of the risk analysis and, where appropriate, a list of the standards referred to in Article 5, applied in full or in part, and descriptions of the solutions adopted to meet the essential requirements of the Directive if the standards referred to in Article 5 have not been applied in full,
in the case of sterile products or products with a special microbiological state or state of cleanliness, a description of the procedures used,
the results of the design calculations and of the inspections carried out, etc.,
if the device is to be combined with other device(s) in order to operate as intended, proof must be provided that it conforms to the essential requirements when combined with any such device(s) having the characteristics specified by the manufacturer,
the test reports,
adequate performance evaluation data showing the performances claimed by the manufacturer and supported by a reference measurement system (when available), with information on the reference methods, the reference materials, the known reference values, the accuracy and measurement units used; such data should originate from studies in a clinical or other appropriate environment or result from relevant biographical references,
the labels and instructions for use,
the results of stability studies.
4.The manufacturer shall take necessary measures to ensure that the manufacturing process follows the principles of quality assurance as appropriate for the products manufactured.U.K.
The system shall address:
the organisational structure and responsibilities,
the manufacturing processes and systematic quality control of production,
the means to monitor the performance of the quality system.
5.The manufacturer shall institute and keep up to date a systematic procedure to review experience gained from devices in the post-production phase and to implement appropriate means to apply any necessary corrective actions, taking account of the nature and risks in relation to the product. He shall notify the competent authorities of the following incidents immediately on learning of them:U.K.
any malfunction, failure or deterioration in the characteristics and/or performance of a device, as well as any inadequacy in the labelling or the instructions for use which, directly or indirectly, might lead to, or might have led to, the death of a patient or user or other persons or to a serious deterioration in his or their state of health;
any technical or medical reason connected with the characteristics or the performance of a device for the reasons referred to in subparagraph (i) leading to systematic recall of devices of the same type by the manufacturer.
6.For devices for self-testing the manufacturer shall lodge an application for examination of the design with a notified body.U.K.
6.1.The application shall enable the design of the device to be understood and shall enable conformity with the design-related requirements of the directive to be assessed.U.K.
It shall include:
test reports including, where appropriate, results of studies carried out with lay persons,
data showing the handling suitability of the device in view of its intended purpose for self-testing,
the information to be provided with the device on its label and its instructions for use.
6.2.The notified body shall examine the application and, if the design conforms to the relevant provisions of this Directive shall issue the applicant with an EC design-examination certificate. The notified body may require the application to be completed by further tests or proof to allow assessment of conformity with the design-related requirements of the Directive. The certificate shall contain the conclusions of the examination, the conditions of validity, the data needed for identification of the approved design and, where appropriate, a description of the intended purpose of the product.U.K.
6.3.The applicant shall inform the notified body which issued the EC design-examination certificate of any significant change made to the approved design. Changes to the approved design must receive further approval from the notified body which issued the EC design-examination certificate wherever the changes could affect conformity with the essential requirements of the Directive or with the conditions prescribed for use of the product. This additional approval shall take the form of a supplement to the EC design-examination certificate.U.K.
ANNEX IVU.K.EC DECLARATION OF CONFORMITY(FULL QUALITY ASSURANCE SYSTEM)
1.The manufacturer must ensure application of the quality system approved for the design, manufacture and final inspection of the devices concerned, as specified in section 3, and is subject to audit as laid down in section 3.3 and to the surveillance as specified in section 5. In addition, the manufacturer must follow, for devices covered by Annex II, List A, the procedures laid down in sections 4 and 6.U.K.
2.The declaration of conformity is the procedure whereby the manufacturer who fulfils the obligations imposed by section 1 ensures and declares that the devices concerned meet the provisions of this Directive which apply to them.U.K.
The manufacturer shall affix the CE marking in accordance with Article 16 and shall draw up a declaration of conformity covering the devices concerned.
3.Quality systemU.K.
3.1.The manufacturer must lodge an application for assessment of his quality system with a notified body.U.K.
The application must include:
the name and address of the manufacturer and any additional manufacturing site covered by the quality system,
adequate information on the device or device category covered by the procedure,
a written declaration that no such application has been lodged with any other notified body for the same device-related quality system,
the documentation on the quality system,
an undertaking by the manufacturer to fulfil the obligations imposed by the quality system approved,
an undertaking by the manufacturer to keep the approved quality system adequate and efficacious,
an undertaking by the manufacturer to institute and keep up to date a systematic procedure to review experience gained from devices in the post-production phase and to implement appropriate means to apply any necessary corrective action and notification as referred to in Annex III, section 5.
3.2.Application of the quality system must ensure that the devices conform to the provisions of this Directive which apply to them at every stage, from design to final inspection. All the elements, requirements and provisions adopted by the manufacturer for his quality system must be documented in a systematic and orderly manner in the form of written policies and procedures, such as quality programmes, quality plans, quality manuals and quality records.U.K.
It shall include in particular an adequate description of:
the manufacturer's quality objectives;
the organisation of the business and in particular:
the organisational structures, the responsibilities of the managerial staff and their organisational authority where quality of design and manufacture of the devices is concerned,
the methods of monitoring the efficient operation of the quality system and in particular its ability to achieve the desired quality of design and of product, including control of devices which fail to conform;
the procedures for monitoring and verifying the design of the devices and in particular:
a general description of the device, including any variants planned,
all documentation referred to in Annex III, section 3, indents 3 to 13,
in the case of devices for self-testing, the information referred to in Annex III, section 6.1,
the techniques used to control and verify the design and the processes and systematic measures which will be used when the devices are being designed;
the inspection and quality assurance techniques at the manufacturing stage and in particular:
the processes and procedures which will be used, particularly as regards sterilisation,
the procedures in relation to purchasing,
the product identification procedures drawn up and kept up to date from drawings, specifications or other relevant documents at every stage of manufacture;
the appropriate tests and trials which will be carried out before, during and after manufacture, the frequency with which they will take place, and the test equipment used; it must be possible to trace back the calibration.
The manufacturer shall carry out the required controls and tests according to the latest state of the art. The controls and tests shall cover the manufacturing process including the characterisation of the raw material and the individual devices or each batch of devices manufactured.
In testing the devices covered by Annex II, List A, the manufacturer shall take into account the most recent available information, in particular as regards the biological complexity and variability of the specimens to be tested with the in vitro device concerned.
3.3.The notified body must audit the quality system to determine whether it meets the requirements referred to in section 3.2. It must presume that quality systems which implement the relevant harmonised standards conform to the requirements.U.K.
The assessment team must have experience of assessments of the technology concerned. The assessment procedure must include an inspection on the manufacturer's premises and, in duly substantiated cases, on the premises of the manufacturer's suppliers and/or subcontractors to inspect the manufacturing processes.
The decision shall be notified to the manufacturer. It must contain the conclusions of the inspection and a reasoned assessment.
3.4.The manufacturer must inform the notified body which approved the quality system of any plan for substantial changes to the quality system or the product-range covered.U.K.
The notified body must assess the changes proposed and verify whether after these changes the quality system still meets the requirements referred to in section 3.2. It must notify the manufacturer of its decision. This decision must contain the conclusions of the inspection and a reasoned assessment.
4.Examination of the design of the productU.K.
4.1.For devices covered by Annex II, List A, in addition to the obligations imposed by section 3, the manufacturer must lodge with the notified body an application for examination of the design dossier relating to the device which he plans to manufacture and which falls into the category referred to in section 3.1.U.K.
4.2.The application must describe the design, manufacture and performances of the device in question. It must include the documents needed to assess whether the device conforms to the requirements of this Directive, as referred to in section 3.2(c).U.K.
4.3.The notified body must examine the application and, if the device conforms to the relevant provisions of the Directive, issue the application with an EC design-examination certificate. The notified body may require the application to be completed by further tests or proof to allow assessment of conformity with the requirements of the Directive. The certificate must contain the conclusions of the examination, the conditions of validity, the data needed for the identification of the approved design and, where appropriate, a description of the intended purpose of the device.U.K.
4.4.Changes to the approved design must receive further approval from the notified body which issued the EC design-examination certificate wherever the changes could affect conformity with the essential requirements of the Directive or with the conditions prescribed for use of the device. The applicant shall inform the notified body which issued the EC design-examination certificate of any such changes made to the approved design. The additional approval must take the form of a supplement to the EC design-examination certificate.U.K.
4.5.The manufacturer shall inform the notified body without delay if it has obtained information about changes to the pathogen and markers of infections to be tested, in particular as a consequence of biological complexity and variability. In this connection, the manufacturer shall inform the notified body whether any such change is likely to affect the performance of the in vitro diagnostic medical device concerned.U.K.
5.SurveillanceU.K.
5.1.The aim of surveillance is to ensure that the manufacturer duly fulfils the obligations imposed by the approved quality system.U.K.
5.2.The manufacturer must authorise the notified body to carry out all the necessary inspections and supply it with all relevant information, in particular:U.K.
the documentation on the quality system,
the data stipulated in the part of the quality system relating to design, such as the results of analyses, calculation, tests, etc.,
the data stipulated in the part of the quality system relating to manufacture, such as inspection reports and test data, calibration data, qualification reports of the personnel concerned, etc.
5.3.The notified body must periodically carry out appropriate inspections and assessments to make sure that the manufacturer applies the approved quality system and must supply the manufacturer with an assessment report.U.K.
5.4.In addition, the notified body may pay unannounced visits to the manufacturer. At the time of such visits, the notified body may, where necessary, carry out or ask for tests in order to check that the quality system is working properly. It must provide the manufacturer with an inspection report and, if a test has been carried out, with a test report.U.K.
6.Verification of manufactured products covered by Annex II, List AU.K.
6.1.In the case of devices covered by Annex II, List A, the manufacturer shall forward to the notified body without delay after the conclusion of the controls and tests the relevant reports on the tests carried out on the manufactured devices or each batch of devices. Furthermore, the manufacturer shall make the samples of manufactured devices or batches of devices available to the notified body in accordance with pre-agreed conditions and modalities.U.K.
6.2.The manufacturer may place the devices on the market, unless the notified body communicates to the manufacturer within the agreed time-frame, but not later than 30 days after reception of the samples, any other decision, including in particular any condition of validity of delivered certificates.U.K.
ANNEX VU.K.EC TYPE-EXAMINATION
1.EC type-examination is the part of the procedure whereby a notified body ascertains and certifies that a representative sample of the production envisaged fulfils the relevant provisions of this Directive.U.K.
2.The application for EC type-examination shall be lodged by the manufacturer or by his authorised representative with a notified body.U.K.
The application shall include:
the name and the address of the manufacturer and the name and address of the authorised representative if the application is lodged by the representative,
the documentation described in section 3 needed to assess the conformity of the representative sample of the production in question, hereinafter referred to as the ‘type’, with the requirements of this Directive. The applicant shall make a ‘type’ available to the notified body. The notified body may request other samples as necessary,
a written declaration that no application has been lodged with any other notified body for the same type.
3.The documentation must allow an understanding of the design, the manufacture and the performances of the device. The documentation shall contain the following items in particular:U.K.
a general description of the type, including any variants planned,
all documentation referred to in Annex III, section 3, indents 3 to 13,
in the case of devices for self testing, the information referred to in Annex III, section 6.1.
4.The notified body shall:U.K.
4.1.examine and assess the documentation and verify that the type has been manufactured in conformity with that documentation; it shall also record the items designed in conformity with the applicable provisions of the standards referred to in Article 5, as well as the items not designed on the basis of the relevant provisions of the abovementioned standards;U.K.
4.2.perform or have performed appropriate examinations and the tests necessary to verify whether the solutions adopted by the manufacturer meet the essential requirements of this Directive if the standards referred to in Article 5 have not been applied; if the device is to be combined with other device(s) in order to operate as intended, proof must be provided that it conforms to the essential requirements when combined with any such device(s) having the characteristics specified by the manufacturer;U.K.
4.3.carry out or ask for the appropriate examinations and the tests necessary to verify whether, if the manufacturer has chosen to apply the relevant standards, these have actually been applied;U.K.
4.4.agree with the applicant on the place where the necessary examinations and tests will be carried out.U.K.
5.If the type conforms to the provisions of this Directive, the notified body shall issue the applicant with an EC type-examination certificate. The certificate shall contain the name and address of the manufacturer, the conclusions of the examination, the conditions of validity and the data needed for identification of the type approved. The relevant parts of the documentation shall be annexed to the certificate and a copy shall be kept by the notified body.U.K.
6.The manufacturer shall inform the notified body without delay if it has obtained information about changes to the pathogen and markers of infections to be tested, in particular as a consequence of biological complexity and variability. In this connection, the manufacturer shall inform the notified body whether any such change is likely to affect the performance of the in vitro device concerned.U.K.
6.1.Changes to the approved device must receive further approval from the notified body which issued the EC type-examination certificate wherever the changes may affect conformity with the essential requirements of the Directive or with the conditions prescribed for use of the device. The applicant shall inform the notified body which issued the EC type-examination certificate of any such change made to the approved device. This new approval shall take the form of a supplement to the initial EC type-examination certificate.U.K.
7.Administrative provisionsU.K.
Other notified bodies may obtain a copy of the EC type-examination certificates and/or the supplements thereto. The annexes to the certificates must be available to the other notified bodies on reasoned application, after the manufacturer has been informed.
ANNEX VIU.K.EC VERIFICATION
1.EC verification is the procedure whereby the manufacturer or his authorised representative ensures and declares that the products which have been subject to the procedure set out in section 4 conform to the type described in the EC type-examination certificate and meet the requirements of this Directive which apply to them.U.K.
2.1.The manufacturer must take all the measures necessary to ensure that the manufacturing process produces products which conform to the type described in the EC type-examination certificate and the requirements of the Directive which apply to them. Before the start of manufacture, the manufacturer must prepare documents defining the manufacturing process, in particular as regards sterilisation and the suitability of starting materials, where necessary, and define the necessary testing procedures according to the state of the art. All the routine, pre-established provisions must be implemented to ensure homogeneous production and conformity of the products with the type described in the EC type-examination certificate and with the requirements of this Directive which apply to them.U.K.
2.2.To the extent that for certain aspects the final testing according to section 6.3 is not appropriate, adequate process testing, monitoring and control methods shall be established by the manufacturer with the approval of the notified body. The provisions of Annex IV, section 5, shall apply accordingly in relation to the abovementioned approved procedures.U.K.
3.The manufacturer must undertake to institute and keep up to date a systematic procedure to review experience gained from devices in the post-production phase and to implement appropriate means to apply any necessary corrective and notification action as referred to in Annex III, section 5.U.K.
4.The notified body must carry out the appropriate examinations and tests taking account of section 2.2 in order to verify the conformity of the product with the requirements of the Directive either by examining and testing every product as specified in section 5 or by examining and testing products on a statistical basis as specified in section 6, as the manufacturer decides. When carrying out statistical verification according to section 6, the notified body has to decide when statistical procedures for lot-by-lot inspection or isolated lot inspection have to be applied. Such decision must be taken in consultation with the manufacturer.U.K.
In as far as the conduct of examinations and tests on a statistical basis is not appropriate, examinations and tests may be carried out on a random basis provided that such procedure in conjunction with the measures taken in accordance with section 2.2 ensures an equivalent level of conformity.
5.Verification by examination and testing of every productU.K.
5.1.Every product is examined individually and the appropriate tests defined in the relevant standard(s) referred to in Article 5 or equivalent tests must be carried out in order to verify the conformity of the products with the EC type described in the type-examination certificate and with the requirements of the Directive which apply to them.U.K.
5.2.The notified body must affix, or have affixed, its identification number to each approved product and must draw up a written certificate of conformity relating to the tests carried out.U.K.
6.Statistical verificationU.K.
6.1.The manufacturer must present the manufactured products in the form of homogeneous batches.U.K.
6.2.One or more random samples, as necessary, are taken from each batch. The products which make up the sample are examined and the appropriate tests defined in the relevant standard(s) referred to in Article 5 or equivalent tests must be carried out to verify, where appropriate, the conformity of the products with the type described in the EC type-examination certificate and with the requirements of the Directive which apply to them in order to determine whether to accept or reject the batch.U.K.
6.3.Statistical control of products will be based on attributes and/or variables, entailing sampling schemes with operational characteristics which ensure a high level of safety and performance according to the state of the art. The sampling scheme will be established by the harmonised standards referred to in Article 5, taking account of the specific nature of the product categories in question.U.K.
6.4.If the batch is accepted, the notified body affixes, or has affixed its identification number to each product and draws up a written certificate of conformity relating to the tests carried out. All products in the batch may be put on the market except any in the sample which failed to conform.U.K.
If the batch is rejected the competent notified body must take appropriate measures to prevent the batch from being placed on the market. In the event of frequent rejection of batches, the notified body may suspend the statistical verification.
The manufacturer may, on the responsibility of the notified body, affix the notified body's identification number during the manufacturing process.
ANNEX VIIU.K.EC DECLARATION OF CONFORMITY(PRODUCTION QUALITY ASSURANCE)
1.The manufacturer must ensure application of the quality system approved for the manufacture of the devices concerned and carry out the final inspection, as specified in section 3, and is subject to the surveillance referred to in section 4.U.K.
2.The declaration of conformity is the part of the procedure whereby the manufacturer who fulfils the obligations imposed by section 1 ensures and declares that the products concerned conform to the type described in the EC type-examination certificate and meet the provisions of this Directive which apply to them.U.K.
The manufacturer must affix the CE marking in accordance with Article 16 and draw up a declaration of conformity covering the devices concerned.
3.Quality systemU.K.
3.1.The manufacturer must lodge an application for assessment of his quality system with a notified body.U.K.
The application must include:
the technical documentation on the types approved and a copy of the EC type-examination certificates.
3.2.Application of the quality system must ensure that the devices conform to the type described in the EC type-examination certificate.U.K.
All the elements, requirements and provisions adopted by the manufacturer for his quality system must be documented in a systematic and orderly manner in the form of written policy statements and procedures. This quality system documentation must permit uniform interpretation of the quality policy and procedures such as quality programmes, plans, manuals and records.
It must include in particular an adequate description of:
the manufacturer's quality objectives;
the organisation of the business and in particular:
the organisational structures, the responsibilities of the managerial staff and their organisational authority where quality of manufacture of the devices is concerned,
the methods of monitoring the efficient operation of the quality system and in particular its ability to achieve the desired quality of product, including control of devices which fail to conform;
the inspection and quality assurance techniques at the manufacturing stage and in particular:
the processes and procedures which will be used, particularly as regards sterilisation,
the procedures in relation to purchasing,
the product identification procedures drawn up and kept up to date from drawings, specifications or other relevant documents at every stage of manufacture;
the appropriate tests and trials to be carried out before, during and after manufacture, the frequency with which they will take place, and the test equipment used; it must be possible to trace back the calibration.
3.3.The notified body must audit the quality system to determine whether it meets the requirements referred to in section 3.2. It must presume that quality systems which implement the relevant harmonised standards conform to these requirements.U.K.
The assessment team must have past experience of assessments of the technology concerned. The assessment procedure must include an inspection on the manufacturer's premises and, in duly substantiated cases, on the premises of the manufacturer's suppliers and/or subcontractors to inspect the manufacturing processes.
The decision must be notified to the manufacturer. It must contain the conclusions of the inspection and a reasoned assessment.
3.4.The manufacturer shall inform the notified body which approved the quality system of any plan for substantial changes to the quality system.U.K.
The notified body must assess the changes proposed and verify whether after these changes the quality system still meets the requirements referred to in section 3.2. It must notify the manufacturer of its decision. This decision must contain the conclusions of the inspection and a reasoned assessment.
4.SurveillanceU.K.
The provision of Annex IV, section 5, shall apply.
5.Verification of manufactured products covered by Annex II, List AU.K.
5.1.In the case of devices covered by Annex II, List A, the manufacturer shall forward to the notified body without delay after the conclusion of the controls and tests the relevant reports on the tests carried out on the manufactured devices or each batch of devices. Furthermore, the manufacturer shall make the samples of manufactured devices or batches of devices available to the notified body in accordance with pre-agreed conditions and modalities.U.K.
5.2.The manufacturer may place the devices on the market, unless the notified body communicates to the manufacturer within the agreed time-frame, but not later than 30 days after reception of the samples, any other decision, including in particular any condition of validity of delivered certificates.U.K.
ANNEX VIIIU.K.STATEMENT AND PROCEDURES CONCERNING DEVICES FOR PERFORMANCE EVALUATION
1.For devices for performance evaluation the manufacturer or his authorised representative shall draw up the statement containing the information stipulated in section 2 and ensure that the relevant provisions of this Directive are met.U.K.
2.The statement shall contain the following information:U.K.
data allowing identification of the device in question,
an evaluation plan stating in particular the purpose, scientific, technical or medical grounds, scope of the evaluation and number of devices concerned,
the list of laboratories or other institutions taking part in the evaluation study,
the starting date and scheduled duration for the evaluations and, in the case of devices for self-testing, the location and number of lay persons involved,
a statement that the device in question conforms to the requirements of the Directive, apart from the aspects covered by the evaluation and apart from those specifically itemised in the statement, and that every precaution has been taken to protect the health and safety of the patient, user and other persons.
3.The manufacturer shall also undertake to keep available for the competent national authorities the documentation allowing an understanding of the design, manufacture and performances of the product, including the expected performances, so as to allow assessment of conformity with the requirements of this Directive. This documentation must be kept for a period ending at least five years after the end of the performance evaluation.U.K.
The manufacturer shall take all the measures necessary for the manufacturing process to ensure that the products manufactured conform to the documentation mentioned in the first paragraph.
4.The provisions of Article 10(1), (3) and (5) shall apply to devices intended for performance evaluation.U.K.
ANNEX IXU.K.CRITERIA FOR THE DESIGNATION OF NOTIFIED BODIES
1.The notified body, its director and the assessment and verification staff shall not be the designer, manufacturer, supplier, installer or user of the devices which they inspect, nor the authorised representative of any of these persons. They may not be directly involved in the design, construction, marketing or maintenance of the devices, nor represent the parties engaged in these activities. This in no way precludes the possibility of exchanges of technical information between the manufacturer and the body.U.K.
2.The notified body and its staff must carry out the assessment and verification operations with the highest degree of professional integrity and the requisite competence in the field of medical devices and must be free from all pressures and inducements, particularly financial, which might influence their judgment or the results of the inspection, especially from persons or groups of persons with an interest in the results of the verifications.U.K.
Should the notified body subcontract specific tasks connected with the establishment and verification of the facts, it must first ensure that the subcontractor meets the provisions of the Directive. The notified body shall keep at the disposal of the national authorities the relevant documents assessing the subcontractor's qualifications and the work carried out by the subcontractor under this Directive.
3.The notified body must be able to carry out all the tasks assigned to such bodies by one of Annexes III to VII and for which it has been notified, whether these tasks are carried out by the body itself or on its responsibility. In particular, it must have the necessary staff and possess the facilities needed to perform properly the technical and administrative tasks entailed in assessment and verification. This includes the availability of sufficient scientific staff within the organisation who possess adequate experience and knowledge necessary to assess the biological and medical functionality and performance of devices for which it has been notified, in relation to the requirements of this Directive and, in particular, with Annex I requirements. The notified body must also have access to the equipment necessary for the verifications required.U.K.
4.The inspection staff must have:U.K.
sound vocational training covering all the assessment and verification operations for which the body has been designated,
satisfactory knowledge of the rules on the inspections which they carry out and adequate experience of such inspections,
the ability required to draw up the certificates, records and reports to demonstrate that the inspections have been carried out.
5.The impartiality of the inspection staff must be guaranteed. Their remuneration must not depend on the number of inspections carried out, nor on the results of the inspections.U.K.
6.The body must take out civil liability insurance, unless liability is assumed by the State under domestic legislation or the Member State itself carries out the inspections directly.U.K.
7.The staff of the inspection body are bound to observe professional secrecy with regard to all information gained in the course of their duties (except vis-à-vis the competent administrative authorities of the State in which their activities are carried out) under this Directive or any provision of national law putting it into effect.U.K.
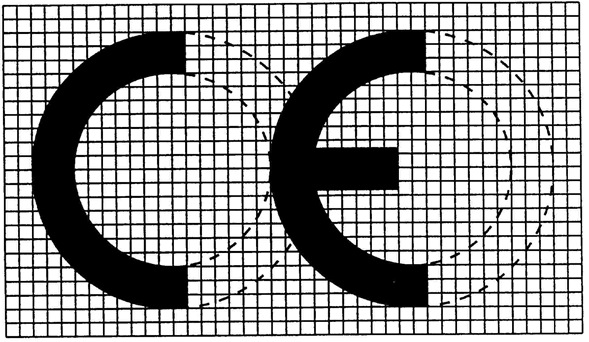
ANNEX XU.K.CE MARKING OF CONFORMITY
If the marking is reduced or enlarged the proportions given in the above graduated drawing must be respected,
the various components of the CE marking must have substantially the same vertical dimension, which may not be less than 5 mm. This minimum dimension may be waived for small-scale devices.
OJ L 39, 15.2.1980, p. 40. Directive as last amended by Directive 89/617/EEC (OJ L 357, 7.12.1989, p. 28).
Council Directive 67/548/EEC of 27 June 1967 on the approximation of laws, regulations and administrative provisions relating to the classification, packaging and labelling of dangerous substances (OJ L 196, 16.8.1967, p. 1). Directive as last amended by Commission Directive 97/69/EC (OJ L 343, 13.12.1997, p. 19).
Council Directive 88/379/EEC of 7 June 1988 on the approximation of the laws, regulations and administrative provisions of the Member States relating to the classification, packaging and labelling of dangerous preparations (OJ L 187, 16.7.1988, p. 14). Directive as last amended by Commission Directive 96/65/EC (OJ L 265, 18.10.1996, p. 15).
Options/Help
Print Options
PrintThe Whole Directive
PrintThe Annexes only
Mae deddfwriaeth ar gael mewn fersiynau gwahanol:
Y Diweddaraf sydd Ar Gael (diwygiedig):Y fersiwn ddiweddaraf sydd ar gael o’r ddeddfwriaeth yn cynnwys newidiadau a wnaed gan ddeddfwriaeth ddilynol ac wedi eu gweithredu gan ein tîm golygyddol. Gellir gweld y newidiadau nad ydym wedi eu gweithredu i’r testun eto yn yr ardal ‘Newidiadau i Ddeddfwriaeth’.
Gwreiddiol (Fel y’i mabwysiadwyd gan yr UE): Mae'r wreiddiol version of the legislation as it stood when it was first adopted in the EU. No changes have been applied to the text.
Gweler y wybodaeth ychwanegol ochr yn ochr â’r cynnwys
Rhychwant ddaearyddol: Indicates the geographical area that this provision applies to. For further information see ‘Frequently Asked Questions’.
Dangos Llinell Amser Newidiadau: See how this legislation has or could change over time. Turning this feature on will show extra navigation options to go to these specific points in time. Return to the latest available version by using the controls above in the What Version box.
Rhagor o Adnoddau
Gallwch wneud defnydd o ddogfennau atodol hanfodol a gwybodaeth ar gyfer yr eitem ddeddfwriaeth o’r tab hwn. Yn ddibynnol ar yr eitem ddeddfwriaeth sydd i’w gweld, gallai hyn gynnwys:
- y PDF print gwreiddiol y fel adopted version that was used for the EU Official Journal
- rhestr o newidiadau a wnaed gan a/neu yn effeithio ar yr eitem hon o ddeddfwriaeth
- pob fformat o’r holl ddogfennau cysylltiedig
- slipiau cywiro
- dolenni i ddeddfwriaeth gysylltiedig ac adnoddau gwybodaeth eraill
Llinell Amser Newidiadau
Mae’r llinell amser yma yn dangos y fersiynau gwahanol a gymerwyd o EUR-Lex yn ogystal ag unrhyw fersiynau dilynol a grëwyd ar ôl y diwrnod ymadael o ganlyniad i newidiadau a wnaed gan ddeddfwriaeth y Deyrnas Unedig.
Cymerir dyddiadau fersiynau’r UE o ddyddiadau’r dogfennau ar EUR-Lex ac efallai na fyddant yn cyfateb â’r adeg pan ddaeth y newidiadau i rym ar gyfer y ddogfen.
Ar gyfer unrhyw fersiynau a grëwyd ar ôl y diwrnod ymadael o ganlyniad i newidiadau a wnaed gan ddeddfwriaeth y Deyrnas Unedig, bydd y dyddiad yn cyd-fynd â’r dyddiad cynharaf y daeth y newid (e.e. ychwanegiad, diddymiad neu gyfnewidiad) a weithredwyd i rym. Am ragor o wybodaeth gweler ein canllaw i ddeddfwriaeth ddiwygiedig ar Ddeall Deddfwriaeth.
Rhagor o Adnoddau
Defnyddiwch y ddewislen hon i agor dogfennau hanfodol sy’n cyd-fynd â’r ddeddfwriaeth a gwybodaeth am yr eitem hon o ddeddfwriaeth. Gan ddibynnu ar yr eitem o ddeddfwriaeth sy’n cael ei gweld gall hyn gynnwys:
- y PDF print gwreiddiol y fel adopted fersiwn a ddefnyddiwyd am y copi print
- slipiau cywiro
liciwch ‘Gweld Mwy’ neu ddewis ‘Rhagor o Adnoddau’ am wybodaeth ychwanegol gan gynnwys
- rhestr o newidiadau a wnaed gan a/neu yn effeithio ar yr eitem hon o ddeddfwriaeth
- manylion rhoi grym a newid cyffredinol
- pob fformat o’r holl ddogfennau cysylltiedig
- dolenni i ddeddfwriaeth gysylltiedig ac adnoddau gwybodaeth eraill