- Latest available (Revised)
- Original (As adopted by EU)
Commission Regulation (EC) No 65/2004Show full title
Commission Regulation (EC) No 65/2004 of 14 January 2004 establishing a system for the development and assignment of unique identifiers for genetically modified organisms
You are here:
- Regulations originating from the EU
- 2004 No. 65
- Whole Regulation
- Previous
- Next
- Show Geographical Extent(e.g. England, Wales, Scotland and Northern Ireland)
- Show Timeline of Changes
More Resources
When the UK left the EU, legislation.gov.uk published EU legislation that had been published by the EU up to IP completion day (31 December 2020 11.00 p.m.). On legislation.gov.uk, these items of legislation are kept up-to-date with any amendments made by the UK since then.
This item of legislation originated from the EU
Legislation.gov.uk publishes the UK version. EUR-Lex publishes the EU version. The EU Exit Web Archive holds a snapshot of EUR-Lex’s version from IP completion day (31 December 2020 11.00 p.m.).
Changes over time for: Commission Regulation (EC) No 65/2004
Changes to legislation:
There are currently no known outstanding effects by UK legislation for Commission Regulation (EC) No 65/2004.![]()
Changes to Legislation
Revised legislation carried on this site may not be fully up to date. At the current time any known changes or effects made by subsequent legislation have been applied to the text of the legislation you are viewing by the editorial team. Please see ‘Frequently Asked Questions’ for details regarding the timescales for which new effects are identified and recorded on this site.
Commission Regulation (EC) No 65/2004
of 14 January 2004
establishing a system for the development and assignment of unique identifiers for genetically modified organisms
THE COMMISSION OF THE EUROPEAN COMMUNITIES,
Having regard to the Treaty establishing the European Community,
Having regard to Regulation (EC) No 1830/2003, of the European Parliament and of the Council, of 22 September 2003, concerning the traceability and labelling of genetically modified organisms and the traceability of food and feed products produced from genetically modified organisms and amending Directive 2001/18/EC(1), and in particular Article 8 thereof,
Whereas:
(1)
Regulation (EC) No 1830/2003 lays down a harmonised framework for the traceability of genetically modified organisms, hereinafter "GMOs", and of food and feed products produced from GMOs through the transmission and holding of relevant information by operators for such products at each stage of their placing on the market.
(2)
Under that Regulation, an operator placing on the market products containing or consisting of GMOs is required to include, as part of that relevant information, the unique identifier assigned to each GMO as a means of indicating its presence and reflecting the specific transformation event covered by the consent or authorisation for placing that GMO on the market.
(3)
Unique identifiers should be developed in accordance with a particular format in order to ensure consistency both at Community and international level.
(4)
The consent or authorisation granted for the placing on the market of a given GMO under Directive 2001/18/EC of the European Parliament and of the Council of 12 March 2001 on the deliberate release into the environment of genetically modified organisms and repealing Council Directive 90/220/EEC(2) or other Community legislation should specify the unique identifier for that GMO. Moreover, the person applying for such consent should ensure that the application specifies the appropriate unique identifier.
(5)
Where, prior to the entry into force of this Regulation, consents have been granted for the placing on the market of GMOs under Council Directive 90/220/EEC of 23 April 1990 on the deliberate release into the environment of genetically modified organisms(3), it is necessary to ensure that a unique identifier is or has been developed, assigned and appropriately recorded for each GMO covered by those consents.
(6)
In order to take account of and maintain consistency with developments in international fora, it is appropriate to have regard to the formats for unique identifiers established by the Organisation for Economic Cooperation and Development (OECD), for use in the context of its BioTrack product database and in the context of the Biosafety clearing house established by the Cartagena Protocol on Biosafety to the Convention on Biological Diversity.
(7)
For the purposes of the full application of Regulation (EC) No 1830/2003, it is essential that this Regulation apply as a matter of urgency.
(8)
The measures provided for in this Regulation are in accordance with the opinion of the Committee set up under Article 30 of Directive 2001/18/EC,
HAS ADOPTED THIS REGULATION:
CHAPTER IU.K.SCOPE
[F1Article 1U.K.
1.This Regulation applies to genetically modified organisms, hereinafter ‘GMOs’, authorised for placing on the market in accordance with Regulation 1829/2003 of the European Parliament and of the Council or—
(a)in England, the Genetically Modified Organisms (Deliberate Release) Regulations 2002,
(b)in Wales, the Genetically Modified Organisms (Deliberate Release) (Wales) Regulations 2002,
(c)in Scotland, the Genetically Modified Organisms (Deliberate Release) (Scotland) Regulations 2002,
and applications for placing on the market under such legislation.
2. This Regulation does not apply to medicinal products for human and veterinary use authorised under—
(a)Regulation (EC) No 726/2004 of the European Parliament and of the Council,
(b)the Human Medicines Regulations 2012, or
(c)the Veterinary Medicines Regulations 2013,
or applications for authorisation under such legislation.]
Textual Amendments
F1Art. 1 substituted (31.12.2020) by The Genetically Modified Organisms (Amendment) (EU Exit) Regulations 2019 (S.I. 2019/90), regs. 1(2)(b), 6(2) (as amended by S.I. 2019/759, regs. 1(a), 12(3) and S.I. 2020/1421, reg. 1(4), Sch. paras. 4(2), 5(2)); 2020 c. 1, Sch. 5 para. 1(1)
CHAPTER IIU.K.APPLICATIONS FOR THE PLACING ON THE MARKET OF GMOs
Article 2U.K.
1.Applications for the placing on the market of GMOs shall include a unique identifier for each GMO concerned.
2.Applicants shall, in accordance with the formats set out in the Annex, develop the unique identifier for each GMO concerned, following consultation of the OECD BioTrack product database, and the Biosafety clearing house [F2established by the Cartagena Protocol on Biosafety to the Convention on Biological Diversity (the “Biosafety clearing house”)], to determine whether or not a unique identifier has already been developed for that GMO in accordance with these formats.
Textual Amendments
F2Words in Art. 2(2) inserted (31.12.2020) by The Genetically Modified Organisms (Amendment) (EU Exit) Regulations 2019 (S.I. 2019/90), regs. 1(2)(b), 6(3); 2020 c. 1, Sch. 5 para. 1(1)
Article 3U.K.
Where consent or authorisation is granted for the placing on the market of a GMO:
(a)the consent or authorisation shall specify the unique identifier for that GMO;
(b)the [F3Secretary of State] shall ensure that the unique identifier for that GMO is communicated as soon as possible, in writing, to the Biosafety clearing house;
[F4(c)for GMOs authorised under Regulation (EC) No 1829/2003, the unique identifier must be recorded in the register maintained in accordance with Article 28 of that Regulation.]
Textual Amendments
F3Words in Art. 3(b) substituted (31.12.2020) by The Genetically Modified Organisms (Amendment) (EU Exit) Regulations 2019 (S.I. 2019/90), regs. 1(2)(b), 6(4)(a); 2020 c. 1, Sch. 5 para. 1(1)
F4Art. 3(c) substituted (31.12.2020) by The Genetically Modified Organisms (Amendment) (EU Exit) Regulations 2019 (S.I. 2019/90), regs. 1(2)(b), 6(4)(b); 2020 c. 1, Sch. 5 para. 1(1)
CHAPTER IIIU.K.GMOs FOR WHICH CONSENT FOR THEIR PLACING ON THE MARKET HAS BEEN GRANTED PRIOR TO THE ENTRY INTO FORCE OF THIS REGULATION
F5Article 4U.K.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Textual Amendments
F5Arts. 4-6 omitted (31.12.2020) by virtue of The Genetically Modified Organisms (Amendment) (EU Exit) Regulations 2019 (S.I. 2019/90), regs. 1(2)(b), 6(5); 2020 c. 1, Sch. 5 para. 1(1)
F5Article 5U.K.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Textual Amendments
F5Arts. 4-6 omitted (31.12.2020) by virtue of The Genetically Modified Organisms (Amendment) (EU Exit) Regulations 2019 (S.I. 2019/90), regs. 1(2)(b), 6(5); 2020 c. 1, Sch. 5 para. 1(1)
F5Article 6U.K.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Textual Amendments
F5Arts. 4-6 omitted (31.12.2020) by virtue of The Genetically Modified Organisms (Amendment) (EU Exit) Regulations 2019 (S.I. 2019/90), regs. 1(2)(b), 6(5); 2020 c. 1, Sch. 5 para. 1(1)
CHAPTER IVU.K.FINAL PROVISION
Article 7U.K.
This Regulation shall enter into force on the date of its publication in the Official Journal of the European Union.
F6...
Done at Brussels, 14 January 2004.
For the Commission
Margot Wallström
Member of the Commission
Textual Amendments
F6Words in Signature omitted (31.12.2020) by virtue of The Genetically Modified Organisms (Amendment) (EU Exit) Regulations 2019 (S.I. 2019/90), reg. 6(6); 2020 c. 1, Sch. 5 para. 1(1)
F7F8ANNEXU.K.FORMATS FOR UNIQUE IDENTIFIERS
Textual Amendments
F7Words in Annex Section A para. 1 omitted (31.12.2020) by virtue of The Genetically Modified Organisms (Amendment) (EU Exit) Regulations 2019 (S.I. 2019/90), regs. 1(2)(b), 6(7)(a); 2020 c. 1, Sch. 5 para. 1(1)
F8Words in Annex Section B substituted (31.12.2020) by The Genetically Modified Organisms (Amendment) (EU Exit) Regulations 2019 (S.I. 2019/90), regs. 1(2)(b), 6(7)(b) (as amended by S.I. 2020/1421, reg. 1(4), Sch. para. 4(3)); 2020 c. 1, Sch. 5 para. 1(1)
The Annex below defines the format for the unique identifier for plants in Section A and for micro-organisms and animals in Section B.
SECTION A
1.Overall formatU.K.
This Annex provides details as to the format to be used for unique identifiers for GMOs pending or authorised for the placing on the market F7.... The format consists of three components comprising a number of alphanumeric digits and providing reference to the applicant/consent holder, transformation event and a means for verification.
The format comprises nine alphanumeric digits in total. The first component represents the applicant/consent holder and comprises two or three alphanumeric digits. The second component comprises five or six alphanumeric digits and represents the transformation event. The third component provides for verification and is represented by a final numerical digit.
The following provides an example of a unique identifier developed using this format.
or
The following sections provide guidance as to how the three individual components of the unique identifier should be developed.
2.Applicant/consent holder componentU.K.
The first two or three alphanumeric digits represent the applicant/consent holder (for example, the first two or three letters of the applicant/consent holder organisation name), followed by a dash, such;
or
Applicants may already have assigned alphanumeric digits to indicate their identity and these appear in the applicant's code table within the OECD BioTrack product database. These applicants should continue to use these digits.
Any new applicant that is not identified within the database will not be permitted to use the existing codes listed in the applicant's code table within the database. The new applicant Should inform the national authorities, which should update the OECD BioTrack product database by including a new code (digits) that will be designed to identify the new applicant in the code table.
3.Transformation event componentU.K.
The second set of five or six alphanumerical digits should represent the specific transformation event(s), which is the subject of the application for the placing on the market and/or consent, such as:
or
Clearly, an individual transformation event may occur in different organisms, species and varieties and the digits should be representative of the specific event in question. Again, applicants should, prior to formulating unique identifiers, consult the OECD BioTrack product database in terms of the unique identifiers that have been assigned to similar transformation events of the same organism/species in order to provide consistency and to avoid duplication.
Applicants should develop their own internal mechanism to avoid applying the same designation (digits) to a "transformation event" if used in a different organism. Where similar transformation events are developed by two or more organisations, the "applicant information" (see section 2) should enable applicants to generate a unique identifier for their own product, while at the same time ensuring its uniqueness from those generated by other applicants.
As regards new GMOs compromising more than one transformation event (often referred to as stacked-gene transformation events), applicants or consent holders should generate a novel unique identifier for such GMOs.
4.Verification componentU.K.
The final digit of the unique identifier is for verification, which shall be separated from the rest of the unique identifier digits by a dash, such as:
or
The verification digit is intended to reduce errors by ensuring the integrity of the alphanumeric identifier, entered by the users of the database.
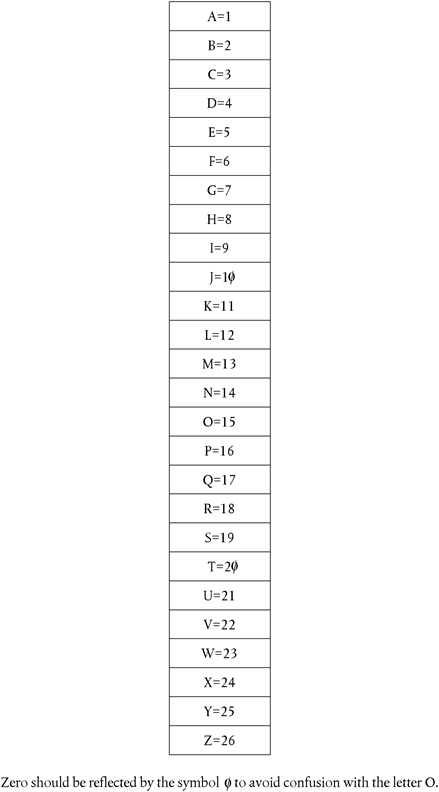
The rule to calculate the verification digit is as follows. The verification digit is made up of a single numerical digit. It is calculated by adding together the numerical values of each of the alphanumerical digits in the unique identifier. The numerical value of each of the digits is from

For example, the verification digit for the code CED-AB891 is calculated as follows:
| step one: | 3 + 5 + 4 + 1 + 2 + 8 + 9 + 1 = 33; |
| step two: | 3 + 3 = 6; therefore the verification digit is 6. |
Therefore, the final unique identifier then becomes - CED-AB891-6.
5.Form of digits to be used in the unique identifierU.K.
6.Form of alphabetic characters to be used, plus numerical equivalents for calculating verification digit.U.K.
SECTION BThe provisions of section A of this Annex shall apply to micro-organisms and animals unless another format for a unique identifier is adopted internationally and [F8implemented in Great Britain].U.K.
OJ L 268, 18.10.2003, p. 24.
OJ L 106, 17.4.2001, p. 1. Directive as last amended by Regulation (EC) No 1830/2003.
OJ L 117, 8.5.1990, p. 15. Directive as last amended by Directive 2001/18/EC.
Options/Help
Print Options
PrintThe Whole Regulation
Legislation is available in different versions:
Latest Available (revised):The latest available updated version of the legislation incorporating changes made by subsequent legislation and applied by our editorial team. Changes we have not yet applied to the text, can be found in the ‘Changes to Legislation’ area.
Original (As adopted by EU): The original version of the legislation as it stood when it was first adopted in the EU. No changes have been applied to the text.
See additional information alongside the content
Geographical Extent: Indicates the geographical area that this provision applies to. For further information see ‘Frequently Asked Questions’.
Show Timeline of Changes: See how this legislation has or could change over time. Turning this feature on will show extra navigation options to go to these specific points in time. Return to the latest available version by using the controls above in the What Version box.
More Resources
Access essential accompanying documents and information for this legislation item from this tab. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as adopted version that was used for the EU Official Journal
- lists of changes made by and/or affecting this legislation item
- all formats of all associated documents
- correction slips
- links to related legislation and further information resources
Timeline of Changes
This timeline shows the different versions taken from EUR-Lex before exit day and during the implementation period as well as any subsequent versions created after the implementation period as a result of changes made by UK legislation.
The dates for the EU versions are taken from the document dates on EUR-Lex and may not always coincide with when the changes came into force for the document.
For any versions created after the implementation period as a result of changes made by UK legislation the date will coincide with the earliest date on which the change (e.g an insertion, a repeal or a substitution) that was applied came into force. For further information see our guide to revised legislation on Understanding Legislation.
More Resources
Use this menu to access essential accompanying documents and information for this legislation item. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as adopted version that was used for the print copy
- correction slips
Click 'View More' or select 'More Resources' tab for additional information including:
- lists of changes made by and/or affecting this legislation item
- confers power and blanket amendment details
- all formats of all associated documents
- links to related legislation and further information resources