ANNEXU.K.
GUIDELINES FOR THE MANAGEMENT OF THE EUROPEAN UNION RAPID INFORMATION SYSTEM ‘RAPEX’ ESTABLISHED UNDER ARTICLE 12 OF DIRECTIVE 2001/95/EC (THE GENERAL PRODUCT SAFETY DIRECTIVE) AND ITS NOTIFICATION SYSTEM U.K.
PART I U.K.SCOPE AND ADDRESSEES OF THE GUIDELINES
1. Scope, objectives and update U.K.
1.1. Scope U.K.
The ‘Guidelines for the management of the European Union Rapid Information System ‘RAPEX’ established under Article 12 of Directive 2001/95/EC on general product safety’ (the ‘Guidelines’) are adopted by the Commission(1) under Article 11(1) and Annex II, point 8, of Directive 2001/95/EC (the ‘GPSD’). The Commission is assisted by an advisory committee composed of the representatives from EU Member States and established under Article 15(3) of the GPSD.
Point 8 of Annex II to the GPSD states that: ‘The Commission shall prepare and regularly update, in accordance with the procedure laid down in Article 15(3), guidelines concerning the management of RAPEX by the Commission and Member States.’.
Article 11 of the GPSD prescribes that Member States should inform the Commission of measures taken which restrict the placing on the market of products — or require their withdrawal or recall — to the extent that such information is not eligible for the type of notification Article 12 of the GPSD provides for, nor does it qualify for any other notification under any specific Community legislation.
Article 22 of Regulation (EC) No 765/2008, provides that, where a Member State takes or intends to take a measure that prevents, restricts or imposes specific conditions on the marketing and use of products posing a serious risk to the health, safety and other relevant public interests of the end-users, it must immediately notify such a measure to the Commission using RAPEX.
Article 23 of Regulation (EC) No 765/2008 provides that Member States must make available to the Commission the information at their disposal, and not already provided under Article 22, on products presenting a (less than serious) risk.
Article 16 of the GPSD provides an obligation for Member States and the Commission to make available to the public information relating to risks to consumer health and safety posed by products. It would therefore be opportune that all information on measures adopted against products posing a risk, insofar as product safety is at stake, are contained in the system intended for this purpose. Member States are therefore encouraged to provide RAPEX with the measures adopted against products posing a risk and entering into the scope of application of the GPSD or Regulation (EC) No 765/2008. The information can be provided directly in RAPEX. In case the information has to be notified in another information system according to Regulation (EC) No 765/2008(2), the Member State can generate a RAPEX notification from within the information system (see Part II, Chapters 1.2(h) and 2.2 of these Guidelines).
Whereas the GPSD applies only to consumer products posing a risk to the health and safety of consumers, Regulation (EC) No 765/2008 applies to consumer products but also professional products covered by EU harmonisation legislation (such as certain medical devices and marine equipment). It also covers a broader scope of risk, in addition to those related to the health and safety of consumers, such as security and environmental risks. Therefore, a risk can concern not only consumers but also, where Regulation (EC) No 765/2008 applies, other ‘end-users’.
Risk Assessment Guidelines of Appendix 6 on Part III are an integral part of the RAPEX Guidelines. They are the instruments that enable determining the level of risk of a product and therefore help to identify the measures to be adopted.
The Risk Assessment Guidelines refer to the level of risk as well as to the possible injuries caused by a single product. The risk assessment for a single product must be accompanied by sound risk management. For example, the risk level for a defective household electrical appliance posing a risk of fire may be only ‘low’, meaning that the probability of a single appliance causing a fatal fire during the lifetime of the appliance is less than one in a million. Nevertheless, if millions of the defective appliances have been placed on the market, it is almost inevitable that fatal fires will occur if appropriate measures are not taken.
Member States(3), applicant countries, countries which are parties to the European Economic Area (EEA) Agreement as well as other non-EU countries and international organisations that are granted access to RAPEX (on the conditions defined in Article 12(4) of the GPSD), participate in the system according to the rules provided for in the GPSD and these Guidelines(4).
1.2. Objectives U.K.
The objectives of these Guidelines are to:
streamline the processes for the notification mechanisms;
set out the notification criteria for the notification mechanisms;
define the content of notifications and follow-up notifications sent under the notification mechanism, in particular what data are required and which forms are to be used;
establish follow-up activities to be taken by Member States upon receipt of a notification and the type of information to be provided;
describe the handling of notifications and follow-up notifications by the Commission;
set deadlines for the various types of action taken under the notification mechanisms;
set out the practical and technical arrangements needed at Commission and Member State level for the notification mechanisms to be employed effectively and efficiently; and
establish risk assessment methods and, in particular, criteria for identifying serious risks.
1.3. Update U.K.
The Guidelines will be regularly updated by the Commission in accordance with the advisory procedure on the basis of experience and new developments in the product safety area.
2. Addressees of the Guidelines U.K.
The Guidelines are addressed to all Member States authorities acting on product safety and participating in the RAPEX network, including market surveillance authorities responsible for monitoring the compliance of products with safety requirements and authorities in charge of external border controls.
3. Products U.K.
3.1. Products covered by these Guidelines U.K.
These Guidelines cover two sets of products: the products covered by the GPSD and the products covered by Regulation (EC) No 765/2008.
3.1.1. Products covered by the GPSD U.K.
Under Article 2(a) of the GPSD, consumer products for the purpose of these Guidelines are:
—
products that are designed and manufactured for and made available to consumers;
—
products that are designed and manufactured for professionals, which are likely, however, under reasonably foreseeable conditions, to be used by consumers. These are products manufactured for professionals that are made available to consumers, who can purchase and operate them without any special knowledge or training, e.g. a power drill, an angle grinder and a table saw designed and manufactured for professionals, but also supplied on the consumer market (i.e. consumers can readily purchase them in shops and operate them on their own without any special training).
Both products intended for consumers and migrating products can be given to consumers free of charge, can be purchased by consumers and can be provided to consumers in the context of a service. All three situations are covered by RAPEX.
According to Article 2 (a) of the GPSD, products provided to consumers in the context of a service are to be considered as including:
products supplied to consumers that are taken away and used outside the premises of a service provider, such as cars and lawn-mowing machines rented or leased in rental shops, and tattoo inks and implants (that are not classified as medical devices) implanted beneath the skin of a consumer by a service provider;
products used on the premises of a service provider, provided that consumers themselves actively operate a product (e.g. start the machine, have the option of stopping it, and affect its operation by changing its position or intensity during use). Sun-beds used in tanning salons and fitness centres are examples of such products. Use of the products by consumers must be active, and involve a significant degree of control. Merely passive use, such as the use of a shampoo by a person whose hair is washed by a hairdresser, or the use of a bus by its passengers, does not qualify as use by consumers.
3.1.2. Products covered by Regulation (EC) No 765/2008 U.K.
Under Regulation (EC) No 765/2008, products for the purpose of RAPEX are to be considered the products according to the scope and definitions contained in Article 15 of the same Regulation whether intended for consumers or for professional users.
3.2. Products not covered by these Guidelines U.K.
These Guidelines do not cover:
Products that are covered by specific and equivalent notification mechanisms established by other EU legislation, notably:
food and feed and other products covered by Regulation (EC) No 178/2002 of the European Parliament and of the Council(6);
medicinal products covered by Directive 2001/83/EC of the European Parliament and of the Council(7), and Directive 2001/82/EC of the European Parliament and of the Council(8);
medical devices covered by Regulation (EU) 2017/745 of the European Parliament and of the Council(9);
active implantable medical devices covered by Council Directive 90/385/EEC(10).
Products that are not covered by the definition of a ‘product’ as laid down in Article 2(a) of the GPSD, notably:
second-hand products or products supplied as antiques or as products to be repaired or reconditioned prior to being used, provided that the supplier clearly informs the person to whom he supplies the product to that effect (Article 2(a) of the GPSD);
equipment used or operated by a professional service provider to supply a service, e.g. equipment on which consumers ride or travel and equipment which is operated by a service provider and not by the consumer (recital 9 of the GPSD);
Products which do not enter into the definition of product contained in Article 15(4) of Regulation (EC) No 765/2008.
4. Measures U.K.
4.1. Types of measures U.K.
Preventive and restrictive measures can be taken in relation to products posing a risk either on the initiative of the economic operator who placed and/or distributed it on the market (‘voluntary measures’), or as ordered by an authority of a Member State competent to monitor the compliance of products with the safety requirements (‘compulsory measures’).
For the purpose of these Guidelines, the compulsory measures and voluntary measures are defined as follows:
:
measures adopted or decided to be adopted by Member State authorities, often in the form of an administrative decision, which oblige an economic operator to take preventive, corrective or restrictive action in relation to a specific product that they made available on the market.
:
preventive and restrictive measures adopted on a voluntary basis by an economic operator, i.e. without any intervention of an authority of a Member State;
recommendations and agreements with economic operators in their respective activities concluded by Member State authorities; this includes agreements which are not in written form and result in preventive or restrictive action taken by economic operators in their respective activities in relation to products posing a serious risk that they made available on the market.
4.2. Categories of measures U.K.
Article 8(1)(b) to (f) of the GPSD provides a list of the different categories of measures that are notifiable under RAPEX when the conditions for notification are fulfilled, including the following measures:
marking a product with appropriate warnings on the risk(s) it may present;
making the marketing of a product subject to prior conditions;
warning consumers and end-users of the risks that could be posed by a product;
temporary ban on the supply, offer to supply and display of a product;
ban on the marketing of a product and any accompanying measures, i.e. measures required to ensure compliance with the ban;
withdrawal of a product from the market;
recall of a product from consumers;
destruction of a withdrawn or recalled product.
For the purpose of RAPEX, the term ‘withdrawal’ is used exclusively for measures aimed at preventing the distribution, display and offer of a product posing a risk to consumers or other end-users, while the term ‘recall’ is used only for measures aimed at achieving the return of such a product that has already been made available to consumers or other end-users by a producer or distributor.
4.3. Requirements of the measures U.K.
Under Article 12(1) of the GPSD and Article 22 of Regulation (EC) No 765/2008 concerning serious risks, both compulsory and voluntary measures are to be notified in RAPEX.
Preventive and restrictive measures adopted on a voluntary basis by an economic operator, i.e. without any intervention of an authority of a Member State concerning a product posing a serious risk and the related preventive or restrictive measures initiated by an economic operator should be immediately notified to the competent authorities of Member States as indicated in Article 5(3) of the GPSD and in Article 22(2) and (3) of Regulation (EC) No 765/2008.
All categories of preventive and restrictive measures taken in relation to the marketing and use of consumer products posing a serious risk to the health and safety of consumers or, in the case of products covered by Regulation (EC) No 765/2008, posing a serious risk to the health, safety or other relevant public interests of the end-users are subject to the notification obligation under RAPEX.
4.4. Exclusion of generally applicable compulsory measures U.K.
Generally applicable acts adopted at national level and aimed at preventing or restricting the marketing and use of (a) generally described category(ies) of consumer products due to the serious risk they pose to the health and safety of consumers should not be notified to the Commission through the RAPEX application. All such national measures that apply to only generally defined categories of products, such as all products in general or all products serving the same purpose — and not to (categories of) products specifically identified by their brand, specific look, producer, trader, model name or number, etc. — are notified to the Commission under Directive (EU) 2015/1535 of the European Parliament and of the Council(11).
5. Risk Levels U.K.
5.1. Serious risk U.K.
Before an authority of a Member State decides to submit a RAPEX notification, it always performs an appropriate risk assessment (see Part III, Appendix 6 of these Guidelines or the complementary EU general risk assessment methodology for products covered by Regulation (EC) No 765/2008(12)) in order to assess whether the product to be notified poses a serious risk to the health and safety of consumers or, in the case of products covered by Regulation (EC) No 765/2008, a serious risk to the health, safety or to other relevant public interests (for example, security or the environment) of the end-users, and thus whether one of the RAPEX notification criteria is met.
5.2. Less than serious risk U.K.
Notifications sent in accordance with Article 11 of the GPSD or Article 23 of Regulation (EC) No 765/2008 are generally considered as notifications for products posing a less than serious risk. Notifications of such products, contrary to notifications for products presenting a serious risk, do not necessarily involve an obligation for follow-up activities by other Member States unless the nature of the product or of the risk so requires (see Part II Chapter 3.4.6.1).
5.3. Risk assessment method U.K.
Part III, Appendix 6 to these Guidelines sets out a risk assessment method that can be used by Member State authorities to assess the level of risks posed by consumer products to the health and safety of consumers and to decide whether a RAPEX notification is necessary. Equally, you may need to consult the complementary EU general risk assessment methodology as referred to in Chapter 5.1 in case the product concerned is covered by Regulation (EC) No 765/2008.
A specific tool (‘RAG’ or Risk Assessment Guidelines(13)) is available on the RAPEX website and in the RAPEX application to perform risk assessments, which takes accounts of the principles provided for in Appendix 6.
5.4. Assessing authority U.K.
The risk assessment is always performed or checked by the authority of a Member State that either carried out the investigation and took appropriate measures, or which monitored the voluntary action taken with regard to a product posing a risk by an economic operator.
Any unclear issues are resolved by the RAPEX Contact Point (see Part II, Chapter 5.1) with the authority responsible before a notification is transmitted through the RAPEX application.
6. Cross-border effects U.K.
6.1. International event U.K.
Under Article 12 of the GPSD and Article 22 of Regulation (EC) No 765/2008, a Member State submits a RAPEX notification only if it considers that the effects of the risk(s) posed by a product go or can go beyond its territory (‘cross-border effects’ or ‘international event’).
In the light of the free movement of products in the internal market, and the fact that products are imported into the EU through different distribution channels and that consumers buy products during stays abroad and via the internet, national authorities are encouraged to interpret the cross-border effects criterion in a fairly broad sense. An Article 12 of the GPSD or Article 22 notification of Regulation (EC) No 765/2008, therefore, is submitted where:
it cannot be excluded that a product posing a risk has been sold in more than one EU Member State; or
it cannot be excluded that a product posing a risk has been sold via the internet; or
the product originates from a third country and is likely to have been imported into the EU through multiple distribution channels.
6.2. Local event U.K.
Measures adopted in relation to a product posing a serious risk that can only have a local effect (‘Local event’) are not notified under Article 12 of the GPSD. This applies in situations where an authority of a Member State has concrete and strong reasons to exclude the possibility that a product has been and or will be made available (by any means) in other Member States, e.g. measures taken with regard to a local product manufactured and distributed only in one Member State. In its evaluation, the authorities of the Member State have to take carefully into consideration the possibility that a product could be sold online or through new emerging distribution channels.
A notification in relation to a product posing a serious risk involving a local event only requires to be submitted to the Commission insofar as it involves information likely to be of interest to Member States from the product safety standpoint, and in particular if they are in response to a new type of risk which has not yet been notified, a new type of risk arising from a combination of products or a new type or category of products.
Such notification is to be submitted under Article 11 with reference to the second subparagraph of Article 11(1), of the GPSD.
PART II U.K.EU RAPID INFORMATION SYSTEM ‘RAPEX’ ESTABLISHED UNDER ARTICLE 12 OF THE GENERAL PRODUCT SAFETY DIRECTIVE
1. Introduction U.K.
1.1. Objectives of RAPEX U.K.
Article 12 of the GPSD establishes an EU Rapid Information System (‘RAPEX’).
RAPEX plays an important role in the area of product safety. It complements other actions taken both at national and at EU level to ensure a high level of product safety in the EU.
RAPEX data helps to:
prevent and restrict the supply of dangerous products;
monitor the effectiveness and consistency of market surveillance and enforcement activities carried out by Member State authorities;
identify needs and provide a basis for action at EU level; and
make for consistent enforcement of the EU product safety requirements and therefore contribute to the smooth functioning of the single market.
1.2. Components of RAPEX U.K.
RAPEX consists of several complementary components, which are crucial for its effective and efficient operation. The most important are:
the legal framework that regulates how the system operates (i.e. the GPSD and the Guidelines);
the online application (‘the RAPEX application’), which allows Member States and the Commission to exchange information rapidly via a web-based platform;
the RAPEX Contact Points network, which consists of the single RAPEX Contact Points responsible for operating RAPEX in all Member States (see Part II, Chapter 5.1);
the national RAPEX networks established in all Member States, which include the RAPEX Contact Point (see Part II, Chapter 5.1) and all the authorities involved in ensuring product safety;
the Commission RAPEX team in the department responsible for the GPSD, which examines and validates documents submitted through the RAPEX application, and maintains and ensures correct operation of RAPEX;
the RAPEX website(14), which provides summaries of RAPEX notifications as well as weekly updates;
RAPEX publications, such as RAPEX statistics, RAPEX annual reports and other promotional materials; and
the interface between RAPEX and ICSMS, which consists on a link between both systems that facilitates the encoding of RAPEX notifications based on investigation data already available in ICSMS. By filling in the appropriate fields in ICSMS, a RAPEX notification can be automatically submitted.
2. Notification criteria U.K.
RAPEX applies to measures which prevent, restrict or impose specific conditions on the marketing and use of products posing a serious risk to the health and safety of consumers or, in the case of products covered by Regulation (EC) No 765/2008, to measures which prevent, restrict or impose specific conditions on the marketing and use of products posing a serious risk to the health, safety or other relevant public interests (for example, security or the environment) of the end-users.
2.1. Mandatory participation in RAPEX: Article 12 of the GPSD and Article 22 of Regulation (EC) No 765/2008 U.K.
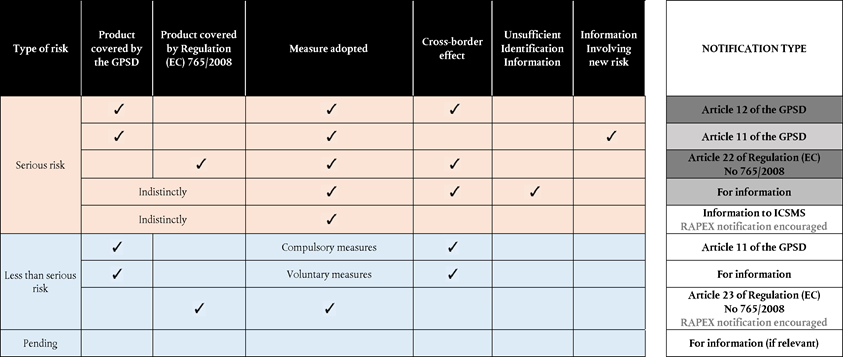
Under the GPSD and Regulation (EC) No 765/2008, the participation of Member States in RAPEX is mandatory. According to Article 12 of the GPSD and Article 22 of Regulation (EC) No 765/2008 Member States have a legal obligation to notify the Commission both compulsory and voluntary measures when the following four notification criteria are met:
the product falls under the scope of application of the GPSD or under the scope of application of Regulation (EC) No 765/2008;
the product is subject to measures that prevent, restrict or impose specific conditions on its possible marketing or use (‘preventive and restrictive measures’);
the product poses a serious risk to the health and safety of consumers or, in case of products covered by Regulation (EC) No 765/2008, also to other relevant public interests of the end-users;
it cannot be ruled out that the effect of the serious risk to the health and safety of consumers or, in case of products covered by Regulation (EC) No 765/2008, also to other relevant public interests of the end-users, goes beyond the territory of the notifying Member State.
2.2. Non-mandatory participation in RAPEX: Article 11 of the GPSD and Article 23 of Regulation (EC) No 765/2008 U.K.
According to Article 11 of the GPSD, Member States should inform the Commission of measures taken which restrict the placing on the market of products — or require their withdrawal or recall — insofar such information does not qualify for an Article 12 nor any other notification set out in any specific Community legislation.
For the sake of simplification and efficiency gains, Member States may also make use of the RAPEX application to notify measures taken against products which would not qualify for submitting an Article 12 notification in the terms outlined herein.
Where the following four notification criteria are met, Member States have a legal obligation to notify the Commission under Article 11 of the GPSD:
the product concerned is a consumer product;
it is subject to restrictive measures adopted by national authorities (compulsory measures);
it poses a less than serious risk to the health and safety of consumers and the effects of which can or do go beyond the territory of one Member State or, it poses a serious risk to the health and safety of consumers and the effect of which do not or cannot go beyond its territory yet the measures adopted involve information likely to be of interest to other Member States from a product safety standpoint(15);
The measures adopted do not have to be notified under any other notification procedure established by EU law.
Notwithstanding the fact that Article 11 of the GPSD does not contain an explicit obligation to notify voluntary measures adopted against products posing a less than serious risk, Article 16 of the GPSD requires Member States and the Commission to make information relating to risks to consumer health and safety available to the public. Therefore, for the sake of coherence in the notification system and to effectively implement the obligations both Member States and the Commission have according to Article 16 of the GPSD, Member States are recommended to notify in RAPEX also voluntary measures adopted by the producers and distributors against products posing a less than serious risk.
According to Article 23 of Regulation (EC) No 765/2008 Member States provide the Commission with information at their disposal, and not already provided under Article 22, on products presenting a (less than serious) risk. Contrary to Article 22 of this Regulation, Article 23 does not oblige Member States to submit a notification to RAPEX with this information. Article 16 of the GPSD obliges, though, the Commission and the Member States to make public the information they may have relating to risks to consumer health and safety. For the sake of coherence and to effectively implement the obligations contained in Article 16 of the GPSD, the most pragmatic solution could be for RAPEX to contain all measures adopted against products presenting serious and less than serious risks to consumer health and safety both for GPSD products and products covered by Regulation (EC) No 765/2008, and in the latter case, also to other relevant public interests of the end-users. Therefore, when measures are adopted and provided through ICSMS according to Article 23 of Regulation (EC) No 765/2008, Member States are encouraged to notify such information in RAPEX. This can be done either by submitting a separate notification in RAPEX or through ICSMS.
A link between both systems facilitates the encoding of notifications based on investigation data already available in ICSMS. (See Part II, Chapter 1.2(h)).
A notification scheme is included in Part III, Appendix 3 of these Guidelines providing further clarification on the notification criteria referred to in Part II Chapter 2 of these Guidelines.
3. Notifications U.K.
3.1. Types of notification U.K.
3.1.1. Notifications U.K.
The Authorities of the Member States are required to submit a notification to the RAPEX system in the following cases:
where all the RAPEX notification criteria laid down in Article 12 of the GPSD(16) are met, a Member State prepares and submits to the Commission a RAPEX notification classified in the RAPEX application as an ‘Article 12 notification’.
where all the RAPEX notification criteria are met and, in addition, a product poses a life-threatening risk and/or there have been fatal accidents, and in other cases where a RAPEX notification requires emergency action by all Member States, the notifying Member State prepares and submits to the Commission a RAPEX notification classified in the RAPEX application as a ‘Notification requiring emergency action’.
where all RAPEX notification criteria laid down in Article 22 of Regulation (EC) No 765/2008(17) are met, a Member State prepares and submits to the Commission a RAPEX notification classified in the RAPEX application as an ‘Article 22 notification’.
Where all notification criteria laid own in Article 11 of the GPSD(18) are met, a Member State prepares and submits to the Commission a notification, which, when notified in RAPEX is classified as an ‘Article 11 notification’.
Moreover, Member States are encouraged to submit a notification where the criteria laid down in Article 23 of Regulation (EC) No 765/2008 are met(19).
Following the abovementioned reasoning in Part II Chapter 2, Member States are encouraged to prepare and submit, either directly or indirectly, to the Commission a notification classified in RAPEX as an ‘Article 23 notification’ when the criteria laid down in the same article are met.
Before sending a notification to the Commission, the RAPEX Contact Point (see Part II, Chapter 5.1) of the notifying Member State checks that all notification criteria are met.
3.1.2. Notifications for information U.K.
If the criteria laid down in these Guidelines for the notifications listed in Part II Chapters 2.1 and 2.2 of these Guidelines are not met, the RAPEX Contact Point (see Part II, Chapter 5.1) may choose to use the RAPEX application to send the information concerned for information purposes. Such notifications are classified in RAPEX as ‘Notifications for information’ and they may be sent in the following situations:
Where all the RAPEX notification criteria laid down in Article 12 of the GPSD or in Article 22 of Regulation (EC) No 765/2008 are met but a notification does not contain all the information (mainly on product identification and distribution channels) necessary for other Member States to ensure follow-up(20) to such a notification. A notification where the product name, brand and picture are missing and thus the notified product cannot be correctly identified and it cannot be distinguished from other products of the same category or type that are available on the market, is an example of a notification that can be distributed through the RAPEX application as ‘Notification for information’. Assessment as to whether a notification contains sufficient information for other Member States to ensure follow-up activities is always on a case-by-case basis.
Where a Member State is aware of the fact that a consumer product that is available on the EU market poses a serious risk to the health and safety of consumers or, in the case of products covered by Regulation (EC) 765/2008, is aware of the fact that a consumer or a professional product poses a serious risk to the health and safety or other relevant public interests of the end-users, but preventive and restrictive measures have not yet been taken by the producer or distributor or adopted or decided to be adopted by an authority of a Member State. If information on such a product is distributed through the RAPEX application before measures are taken, the notifying Member State subsequently informs the Commission (as soon as possible and not later than the deadlines specified in Appendix 4 to these Guidelines) of the final decision taken with regard to the notified product (mainly, what type of preventive or restrictive measures were taken or why such measures were not taken). Where the notifying Member State takes measures at a later stage, it informs the Commission, who will update the notification in application of Article 12 of the GPSD or Article 22 of Regulation (EC) No 765/2008.
Where a Member State decides to notify preventive and restrictive measures taken in relation to a consumer product posing a serious risk to the health and safety of consumers which has only local effects (‘local event’). If, however, as explained in Part I, Chapter 6.2, a notification by ‘local event’ involves information on product safety likely to be of interest for other Member States, it should be sent as if it were a notification under Article 11 of the GPSD.
Where a notification concerns a product whose safety aspects (especially the level of risk posed to the health and safety of consumers) are subject to discussion at EU level to ensure a common approach between Member States to risk assessment and/or enforcement action(21).
Where a decision cannot be taken with certainty that one or more of the notification criteria are met, but a notification involves information on product safety likely to be of interest for other Member States.
When sending a ‘Notification for information’, the RAPEX Contact Point (see Part II, Chapter 5.1) clearly states the reasons for so doing.
3.2. Content of notifications U.K.
3.2.1. Scope of data U.K.
Notifications sent to the Commission through the RAPEX application include the following types of data:
Information enabling the notified product to be identified, i.e. product category, product name, brand, model and/or type number, barcode, batch or serial number, customs code, description of the product and its packaging accompanied by pictures showing the product, its packaging and labels. Detailed and accurate product identification is a key element for market surveillance and enforcement, as it allows national authorities to identify the notified product, to distinguish it from other products of the same or similar type or category that are available on the market and to find it on the market and take or agree on appropriate measures.
Information establishing the product's origin, i.e. country of origin, name, address and contact details, such as telephone number and e-mail address, of a manufacturer and exporters. In particular, Member States provide all available information on manufacturers and exporters located in third countries that cooperate closely with the EU on product safety. The following documents are also to be attached to the form where available: copies of orders, sales contracts, invoices, shipping documents, customs declarations, etc. These documents should be transmitted in pdf format or any other format accepted by the application. Detailed information on third country producers allows the Commission to promote more effective enforcement in those countries and helps to reduce the number of products posing a risk to consumers exported into the EU.
Wherever possible, information about where exactly the product has been made available (a major store, local shop or market, online, etc.).
Information on the safety requirements applicable to the notified product, including the reference number and name of the applicable legislation and standards.
A risk description of the notified product, including a description of the results of laboratory or visual tests, test reports and certificates proving non-compliance of the notified product with the safety requirements, a complete risk assessment with conclusions and information on known accidents or incidents (see Part I Chapter 3.3.1 of these Guidelines).
Information on the supply chains of the notified product in the Member States and, in particular, information on the countries of destination, plus information on importers and also, if available, on distributors of the notified product in Europe.
Information on measures taken, in particular, the type (compulsory or voluntary), category (e.g. withdrawal from the market, recall from consumers), scope (e.g. national, local), and date of entry into force and duration of the measure (e.g. permanent, temporary).
Indication of whether a notification, part of it and/or attachment(s) are covered by confidentiality. Requests for confidentiality are always accompanied by a justification clearly stating the reasons for such a request.
Information on whether the product is counterfeit, when available. For this purpose, the Commission will provide Member States with any specific tools available at European level to facilitate the identification of counterfeit products.
Information on reported accidents related to the product, indicating when possible the reasons for the accident (risk related to the use made by the user or inherent to the product).
Additional information on whether the notification has been submitted in the context of a coordinated enforcement activity at European level.
Information on whether the authorities of a Member State envisage sending other notifications related to the same product or similar products. This should be indicated in the original notification.
Member States are encouraged to look for and provide information on the supply chains of the notified product in non-EU countries that cooperate closely with the EU on product safety.
3.2.2. Completeness of data U.K.
Notifications should be as complete as possible. The elements to be contained in the notification are listed in Appendix 1 to these Guidelines and are included in the RAPEX application. All fields of the notification template should be completed with the required data. Where the required information is not available at the time a notification is submitted, this is clearly indicated and explained on the form by the notifying Member State. Once the missing information becomes available, the notifying Member State updates its notification. The updated notification is examined by the Commission before being validated and distributed through the system.
RAPEX Contact Points provide all national authorities that participate in the RAPEX network with instructions on the scope of data required to complete the notification. This helps to ensure that the information provided by these authorities to the RAPEX Contact Point is correct and complete (see Part II, Chapter 5.1).
Where part of the information required by these Guidelines is not yet available, Member States should nonetheless comply with the established deadlines and not delay sending a RAPEX notification on a product that poses a life-threatening risk to the health and safety of consumers or other end-users and/or where a RAPEX notification requires emergency action by Member States.
Before submitting a notification, the RAPEX Contact Point checks (to avoid any unnecessary duplication) that the product concerned has not already been notified through the RAPEX application by another Member State. If the product has already been notified, rather than creating a new notification, the RAPEX Contact Point submits a follow-up notification to the existing notification and provides any additional information that may be relevant for authorities in other Member States, such as additional vehicle identification numbers, a detailed list of importers and distributors, additional test reports, etc. (See also Part II, Chapter 5.1).
3.2.3. Updating of data U.K.
The notifying Member State informs the Commission (as soon as possible and not later than by the deadlines specified in Appendix 4 to these Guidelines) of any developments that require changes to a notification transmitted through the RAPEX application. In particular, Member States inform the Commission of any changes (e.g. following a ruling by a court during an appeal procedure) to the status of the notified measures, to the risk assessment and to new decisions regarding confidentiality.
The Commission examines the information provided by the notifying Member State and updates the information concerned in the RAPEX application and on the RAPEX website, where necessary.
3.2.4. Responsibility for the information transmitted U.K.
Responsibility for the information provided lies with the notifying Member State(22).
The notifying Member State and the national authority responsible ensure that all data provided through the RAPEX application are accurate so as to avoid any confusion with similar products of the same category or type that are available on the EU market.
The authority(ies) involved in the notification procedure (e.g. by performing the risk assessment of the notified product or by providing information on distribution channels) take responsibility for the information provided through the RAPEX application. The RAPEX Contact Point checks and validates all notifications received from the authorities responsible before transmitting them to the Commission (See also Part II, Chapter 5.1).
Any action taken by the Commission, such as examining notifications, validating and distributing them through the RAPEX application and publishing them on the RAPEX website, does not imply any assumption of responsibility for the information transmitted, which remains with the notifying Member State.
3.3. Actors and roles involved in the notification process U.K.
The parties involved in the notification process and their responsibilities therein are the following:
3.3.1. Economic operators U.K.
Economic operators are not directly involved in the submission of notifications in the RAPEX application.
However, in case of a product posing a risk, economic operators shall immediately inform the competent authorities in all Member States where the product was made available. The conditions and details for providing such information are laid down in Annex I to the GPSD.
Such information will be dealt with by the Member State where the notifying producer/distributor is established (‘Main Member State’).
The transmission of information on products posing a risk can be submitted by economic operators through the ‘Product Safety Business Alert Gateway’, a tool available on the RAPEX website (see Part II Chapter 5.3.2). Economic operators should include a detailed description of the risk of the product and can make use of the ‘RAG tool’ available for this purpose (see Part I Chapter 5.3).
Risk assessments carried out by economic operators are not binding on Member State authorities who are responsible for carrying out their own risk assessment. It is therefore possible for an authority of a Member State to come to a different conclusion regarding the risk assessment provided in an alert submitted via the ‘Business Gateway’.
3.3.2. Member States authorities U.K.
Member States authorities notify the Commission through the RAPEX application about both compulsory and voluntary measures taken on their own territory against products posing a risk.
Member States establish the roles for the creation, submission and follow-up of notifications in RAPEX.
3.3.3. Authorities in charge of external border controls U.K.
Measures adopted by the authorities in charge of external border controls that prevent the marketing in the EU of a consumer product posing a serious risk to the health and safety of consumers (e.g. decisions to stop the import at the EU border) should be notified to the Commission through the RAPEX application in the same manner as measures adopted by market surveillance authorities that restrict the marketing or use of a product.
3.3.4. European Commission U.K.
The Commission may inform the RAPEX Contact Points (see Part II, Chapter 5.1) regarding products posing serious risks, imported into or exported from the Community and the European Economic Area(23).
The Commission may transmit information to the Member States about products of EU and non-EU origin posing a risk that, according to the information available, are likely to be on the EU market. This mainly concerns information that the Commission receives from third countries, international organisations, businesses or other rapid alert systems.
This information might be circulated amongst Member States by means other than the RAPEX application.
3.4. Workflow U.K.
3.4.1. Creation of a notification U.K.
3.4.1.1. By a national authority U.K.
According to the national arrangements, different national authorities involved in the RAPEX process (local/regional market surveillance authorities, external border control authorities, etc.) may be allowed to create a notification.
3.4.1.2. By the Commission U.K.
In certain cases, the Commission may create a notification as explained in point 3.3.4.
3.4.2. Submission of notifications to the Commission U.K.
The RAPEX Contact Point is responsible for the submission of all notifications for validation by the Commission. (See Part II, Chapter 5.1).
3.4.3. Examination of notifications by the Commission U.K.
The Commission checks all notifications received through the RAPEX application before transmitting them to Member States to ensure that they are correct and complete.
3.4.3.1. Correctness U.K.
When assessing the correctness of a notification, the Commission checks in particular that:
The notification meets all the relevant requirements set out in the GPSD or in Article 22 of Regulation (EC) No 765/2008 and in these Guidelines;
the notified product has not already been notified (to avoid any unnecessary duplication, including between ICSMS and RAPEX);
the notification submitted for validation by the notifying Member State is classified in accordance with the criteria set out in Part II Chapter 2 of these Guidelines;
the information provided including the risk assessment takes due account of the applicable legislation and the relevant standards;
the correct notification procedure has been used.
3.4.3.2. Completeness U.K.
Once a notification is confirmed as correct, the Commission checks that it is complete. Part II, Chapters 3.2.1 and 3.2.2 of these Guidelines act as a point of reference. Special attention is given to the parts of a notification concerning product identification, risk description, measures, traceability and distribution channels.
The Commission is not responsible for performing a risk assessment of the product, but only for checking that the notification includes an appropriate risk assessment containing all the elements listed in Part II Chapter 3.2.1 of these Guidelines (with the exceptions referred to in point 3.4.3.3). See also Part I Chapter 5.1 of these Guidelines.
3.4.3.3. Validation of notifications without a detailed risk assessment U.K.
Member States should submit a risk assessment for every notification but in certain cases, the Commission may validate notifications that are submitted without a detailed and individual risk assessment:
(a)Notifications of products posing chemical risksU.K.
The risk level of a product may be considered to be serious if it contains a chemical substance either banned or in a concentration above the limit established by European legislation. Therefore, in cases where measures are taken against products containing a chemical substance subject to a restriction contained in EU Legislation, a notification may be submitted without a detailed risk assessment.
(b)Notifications of cosmetic productsU.K.
Validation of notifications that do not include a detailed risk assessment may equally be possible for cosmetic products containing banned or restricted substances, which are backed up by an EU scientific committee opinion supporting that such presence of substances above the established limits poses a risk to the health and safety of consumers. For this specific product sector, other factors (e.g. concentration or time of exposure) may need to be taken into consideration.
Nevertheless, if measures have been taken against a product containing not authorised chemical substances for which no scientific opinion has been issued confirming that the product poses a risk, a proper risk assessment may be required depending on a case-by-case analysis to prove that the product poses a serious or less than serious risk. In cases where the risk assessment is needed, if such risk assessment is not provided, these cases shall only be validated ‘for information’ in RAPEX.
As regards products that are subject to restrictive measures by market surveillance authorities based on the presence of a chemical substance mentioned in the list of ingredients which is subject to restrictions contained in EU Legislation and where there is no scientific data assessing the risk, notifications need to be assessed on a case-by-case basis. In case where the risk assessment is needed, if such risk assessment is not provided, these cases shall only be validated ‘for information’ in RAPEX.
(c)Notification of other productsU.K.
Where there is well-documented evidence that certain features of certain products consistently lead to a specific risk and risk level (e.g., the presence of any drawstrings or functional cords in the head, neck or upper chest on garments intended for young children always implies a serious risk), no further risk assessment is required for that given product.
3.4.3.4. Requests for additional information U.K.
Should, during examination, the Commission have questions regarding a notification, it may suspend validation of the notification and ask the notifying Member State for additional information or clarification. This additional information is provided by the notifying Member State by the deadline specified in the Commission's request for information.
3.4.3.5. Investigation U.K.
Where necessary, the Commission may carry out an investigation to assess the safety of a product. This investigation may be conducted in particular where there are serious doubts as to the risks posed by the product notified via the RAPEX application. These doubts can either arise during the examination of a notification by the Commission, or be brought to the attention of the Commission by a Member State (e.g. through a follow-up notification) or by a third party (e.g. a producer).
As part of such investigations, the Commission may, in particular:
ask any Member State to provide information or clarification;
ask for an independent risk assessment and independent testing (laboratory or visual) of the product under investigation;
consult the Scientific Committees, the Joint Research Centre or any other institution specialising in the safety of consumer products;
convene the GPSD Committee, Consumer Safety Network and/or RAPEX Contact Points meetings, as well as consult the relevant Working Groups to discuss developments in an investigation.
Where an investigation concerns a product notified through the RAPEX application, the Commission may suspend validation of a notification or, where such a notification has already been validated and distributed through the RAPEX application, temporarily remove the overview published on the RAPEX website. After an investigation, and depending on the outcome, the Commission (after consulting the notifying Member State, where necessary) may in particular validate and distribute through the RAPEX application the previously suspended notification, uphold the validated notification in the RAPEX application (with any changes) or permanently withdraw the notification from RAPEX.
The Commission informs all Member States of the following:
its decision to launch an investigation, clearly stating the reasons for its decision;
its decision to close an investigation, presenting its conclusions and changes to the investigated notification(s) (if any);
all the relevant developments during an investigation.
3.4.4. Validation and distribution of notifications U.K.
The Commission validates and distributes through the RAPEX application, by the deadlines specified in Appendix 5 to these Guidelines, all notifications assessed as correct and complete during the examination.
Where, during an examination, a request for additional information or clarification was sent to the notifying Member State (followed by a reminder, if necessary), the Commission may take the following decisions:
where the additional information or clarification requested has been provided, the Commission re-examines the notification and may validate it with the changed classification where necessary (e.g. from a ‘Notification for information’ to an ‘Article 12 notification’) or keep it on hold until further clarification;
where the additional information or clarification requested has not been provided within a specified deadline or it is insufficient, the Commission takes a decision on the basis of the information provided and, depending on the circumstances, may either validate it after changing the classification (e.g. from an ‘Article 12 notification’ to ‘Notification for information’) or decide not to validate it.
Once a common approach to risk assessment and/or enforcement has been agreed between Member States, depending on the circumstances and the views of the Member States, the Commission may take one of the following actions:
keep the notifications concerned in the RAPEX application;
change the classification of the notifications stored in the RAPEX application;
withdraw notifications from RAPEX(24).
3.4.5. Publication of notifications U.K.
3.4.5.1. Disclosure of information as a general rule U.K.
The public has the right to be informed about products posing a risk. To meet this obligation, the Commission publishes overviews of new notifications on the RAPEX website(25).
For external communication reasons, the RAPEX website will in future be called ‘Safety Gate’.
Member States equally provide the public with information in the national languages on products posing a serious risk to consumers and on measures taken to address this risk. Such information may be distributed via the internet, on paper, by electronic media, etc.
The information made available to the public is a summary of a notification and includes in particular the elements which allow the identification of the product, as well as the information about the risks and measures taken to prevent or restrict those risks. The Commission and the Member States may decide to disclose other elements of the notifications to the public, only when this information, due to its nature, is not confidential (professional secrets) and does not need to be protected.
The following notifications are made available on the RAPEX website, in line with the requirements laid down in Article 16 of the GPSD:
notifications submitted falling under the scope of Article 12 of the GPSD;
notifications submitted falling under the scope of Article 22 of Regulation (EC) No 765/2008;
notifications submitted falling under the scope of Article 11 of the GPSD for products posing less than serious risk, the cross-border effect of which has also been recognised. As Chapter 3.4 provides for, the cross-border effect ascertains whether such a scenario is to be notified under Article 11;
notifications submitted falling under the scope of Article 23 of Regulation (EC) No 765/2008 concerning products presenting risks that are less than serious and regardless of whether the measures taken were compulsory or voluntary(26);
notifications submitted for information only if the notifying Member State so requests by ticking the ad hoc box in RAPEX, especially when voluntary measures are adopted and the products concerned are sufficiently identified. The publication of these notifications might need to be considered from the standpoint of securing an appropriate risk management.
3.4.5.2. Exceptions to the general rule U.K.
Member States and the Commission should not disclose to the public any information about a product notified through the RAPEX application if such disclosure undermines the protection of court proceedings, monitoring and investigation activities or professional secrecy, except for information relating to the safety properties of products which must be made public if circumstances so require to protect the health and safety of consumers, or, in case of products covered by Regulation (EC) No 765/2008, also to protect other relevant public interests of the end-users(27).
3.4.5.3. Requests for confidentiality U.K.
A notifying Member State may request confidentiality of a notification. Such a request clearly indicates the part(s) of the notification that should be kept confidential.
Furthermore, each request for confidentiality is accompanied by a justification clearly stating the reasons(28).
Requests for confidentiality are subject to examination by the Commission. The Commission checks that the request is complete (i.e. that it states which parts of the notification are covered by confidentiality and that it contains a justification) and justified (i.e. that it is in line with the provisions of the GPSD and these Guidelines). A decision as to the validity of the request is taken by the Commission after consulting the respective RAPEX Contact Point. (See Part II, Chapter 5.1).
3.4.5.4. Handling of notifications covered by confidentiality U.K.
Article 16(2) of the GPSD states that the protection of professional secrecy or confidentiality shall not prevent the dissemination to the competent authorities of information relevant for ensuring the effectiveness of market monitoring and surveillance activities. Notifications covered partially or fully by confidentiality are examined by the Commission and, after being validated and distributed through the RAPEX application, they are subject to the usual follow-up activities by the Member States. The confidentiality of a notification or parts of it does not prevent it from being handled and distributed through the RAPEX application to the competent national authorities.
The only significant difference in the handling and follow-up procedures is that the Commission and Member States should not disclose any parts of a notification that are confidential to the public. These parts have to remain confidential and thus they should not be published in any form. Member State authorities that receive confidential information through the RAPEX application ensure that it is protected when performing their activities.
3.4.5.5. Withdrawal of request for confidentiality U.K.
The notifying Member State withdraws its request for confidentiality immediately after the authority in that Member State becomes aware that the justification for such a request is no longer valid, and informs the Commission accordingly. The Commission informs all Member States of the withdrawal of confidentiality on receipt of such a request by the notifying Member State.
A notification that is no longer covered by full or partial confidentiality is made available to the public in line with the ‘general rules’ applying to publication of notifications set out in these Guidelines.
3.4.6. Follow-up to notifications U.K.
3.4.6.1. Follow-up to the different types of notification U.K.
Member States ensure appropriate follow-up to ‘Article 12 notifications’, ‘Article 12 notifications requiring emergency action’, notifications under Article 22 of Regulation (EC) No 765/2008 and to information on products posing a risk sent by the Commission (Chapter 3.3.4) as soon as possible and by the deadlines specified in Appendix 4 to these Guidelines at the latest.
Notifications for information as well as notifications under Article 11 of the GPSD and notifications under Article 23 of Regulation (EC) No 765/2008 (notification for less than serious risks) do not require any specific follow-up activities. These notifications often do not contain the data needed for effective and efficient enforcement regarding the notified product (e.g. the notified product and/or measures are not sufficiently identified) or the level of the risk is not considered to be serious.
Although there is no specific need for a follow-up in the referred cases, it is still important that Member States verify whether they disagree with the consideration of the risk as less than serious so they may eventually make a follow-up upon the information of a different risk assessment. Member States are therefore encouraged to ensure follow-up to such notifications where the notified product is likely to have been made available to consumers on their market and product identification allows measures to be taken.
3.4.6.2. Objectives of the follow-up activities U.K.
On receipt of a notification, a Member State examines the information provided in the notification and takes appropriate action in order to:
establish whether the product was marketed on its territory;
assess what preventive or restrictive measures should be taken with regard to the notified product found on its market, taking into account the measures taken by the notifying Member State and any special circumstances that could justify different types of measures or no action being taken;
perform additional risk assessment and testing of the notified product, if necessary;
collect any additional information that may be relevant for other Member States (e.g. information on distribution channels of the notified product in other Member States).
3.4.6.3. Follow-up techniques U.K.
To ensure efficient and effective follow-up, best practice follow-up techniques should be employed by national authorities, including:
(a)Checks on the marketU.K.
National authorities organise regular (planned and random) checks on the market in order to establish whether consumer products notified through the RAPEX application are made available to consumers. When the Member State is mentioned as a country of destination, reinforced checks on the market shall be carried out, notably by contacting the economic operator(s) indicated in the notification.
(b)Cooperation with business associationsU.K.
National authorities provide, when necessary, business associations with overviews of the most recent notifications and enquire whether any of the notified products were produced or distributed by their members. National authorities provide businesses only with summaries of notifications, such as the weekly overviews published on the RAPEX website. Whole notifications should not be transmitted to third parties, as certain information (e.g. details of the risk description or information on distribution channels) is often confidential and should be protected.
(c)Publication of RAPEX data via the internet or other electronic and paper mediaU.K.
National authorities regularly alert consumers and businesses about consumer products notified through the RAPEX application via their websites and/or other media, e.g. referring consumers and business to the RAPEX website. Information published in this way allows consumers to check whether they have and use products posing a risk and often provides the authority with useful feedback.
(d)Online checksU.K.
National authorities regularly perform online checks to try to identify whether products notified via RAPEX are available on online markets. Online check techniques may include web-crawling, data mining, data scraping, etc.
National authorities apply various follow-up techniques in parallel and ideally do not limit their activities to only one of them.
The Member State in which a manufacturer, a representative or an importer of the notified product is established (‘Main Member State’) ensures appropriate follow-up to notifications distributed through the RAPEX application. The ‘Main Member State’ often has better legal and technical means of obtaining information on the notified case, which will help other Member States to undertake effective follow-up activities.
3.4.7. Withdrawal/removal of notifications U.K.
3.4.7.1. Permanent withdrawal of a notification from RAPEX U.K.
Notifications distributed through the RAPEX application are kept in the system for an unlimited period of time. The Commission may, however, in the situations presented in this Chapter, permanently withdraw a notification from RAPEX.
3.4.7.1.1.Situations where withdrawal of a submitted or validated notification is possibleU.K.
There is proof that one or more of the notification criteria(29) are not met and thus a notification is not justified. This concerns cases in particular where it is established that the original risk assessment was performed incorrectly and that the notified product does not pose a risk. It also covers situations where the notified measures were successfully challenged in court or in other proceedings and they are no longer valid.
No measures have been taken with regard to a product notified through the RAPEX application (for information) before it was decided to adopt measures or take action(30).
After a discussion held at EU level, Member States agree that it is not useful to exchange information on certain safety aspects that have been notified through the RAPEX application(31).
There is proof that products covered by a notification are no longer marketed and there is proof that all items that had been made available have already been withdrawn from the market and retrieved in all Member States.
Withdrawal of a notification that has been submitted or validated cannot be requested on the basis of the fact that the notified product has been subject to changes needed for it to comply with all the applicable safety requirements, unless proof is provided that all the products (items) concerned that had been made available have been withdrawn and retrieved in all Member States and that they are no longer marketed.
3.4.7.1.2.Request for permanent or temporary withdrawal by Member StatesU.K.
The Commission may withdraw notifications from RAPEX only at the request of the notifying Member State, as the latter takes full responsibility for the information transmitted through the system. Other Member States, however, are encouraged to inform the Commission of any facts that may justify withdrawal.
3.4.7.1.3.Content of the request for permanent or temporary withdrawalU.K.
Every request for withdrawal is accompanied by a justification stating the reasons and by all available documents supporting those reasons. The Commission examines each request and checks the justification and the supporting documents in particular. The Commission may request additional information, clarification or the opinion of the notifying Member State and/or other Member States before taking any decision.
3.4.7.1.4.Decision to withdrawU.K.
Should, on the basis of the justification provided, the Commission decide to withdraw a notification from RAPEX, it removes it from:
the RAPEX application (or makes it otherwise invisible to all users of the system);
the RAPEX website (if necessary).
The Commission informs all Member States of the withdrawal of a notification by mail or through other equally effective means and, if necessary, also the public by publishing a corrigendum on the RAPEX website.
3.4.7.2. Temporary removal of a notification from the RAPEX website U.K.
3.4.7.2.1.Situations where temporary removal is possibleU.K.
Where justified, the Commission may temporarily remove a notification from the RAPEX website, especially where the notifying Member State suspects that a risk assessment submitted in a notification has been performed incorrectly and thus the notified product may not pose a risk. A notification can be temporarily removed from the RAPEX website until the risk assessment of the notified product has been clarified.
3.4.7.2.2.Request for temporary removal by Member StatesU.K.
The Commission may temporary remove notifications from the RAPEX application only at the request of the notifying Member State, as the latter takes full responsibility for the information transmitted through the application. Other Member States, however, are encouraged to inform the Commission of any facts that may justify such removal.
3.4.7.2.3.Content of the request for temporary removalU.K.
Every request for temporary removal is accompanied by a justification stating the reasons and by all available documents supporting those reasons. The Commission examines each request and checks the justification and the supporting documents in particular. The Commission may request additional information, clarification or the opinion of the notifying Member State and/or other Member States before taking any decision.
3.4.7.2.4.Decision to removeU.K.
Should, on the basis of the justification provided, the Commission decide to remove a notification from the RAPEX website, it informs all Member States by e-mail or by other equally effective means and, if necessary, also the public by publishing a corrigendum on the RAPEX website.
3.4.7.2.5.Re-publishing of a notification temporarily removedU.K.
The notifying Member State immediately informs the Commission when the reasons for the removal of a notification from the RAPEX website are no longer valid. In particular, it informs the Commission of the results of any new risk assessment to enable the Commission to determine whether to maintain a notification in the RAPEX application and to re-publish it on the RAPEX website or to withdraw it permanently from RAPEX (following a request from the notifying Member State).
The Commission may re-publish a notification on the RAPEX website following a justified request from the notifying Member State after the risk assessment has been clarified.
The Commission informs the other Member States of the re-publishing of a notification on the RAPEX website by e-mail or by other equally effective means and also the public by replacing the corrigendum with a new one on the RAPEX website.
3.4.8. Notifications older than ten years U.K.
The Commission will place all notifications older than ten years in a separate section of the RAPEX website. These notifications will still be available for public consultation.
3.5. Timing and deadlines for notifications U.K.
3.5.1. Timing of the notification U.K.
Article 12(1) of the GPSD and Article 22 of Regulation (EC) No 765/2008 require Member States to immediately notify the Commission through the RAPEX application of preventive and restrictive measures concerning products posing serious risks. This provision applies to both compulsory and voluntary measures, although the timing of the notification is different.
(a)Compulsory measuresU.K.
These measures are notified through the RAPEX application immediately after being adopted or after the decision to adopt them has been taken, even if an appeal against them at national level is likely, if they are already under appeal or they are subject to publication requirements.
This approach is consistent with the objective of RAPEX, i.e. to ensure the rapid exchange of information between Member States and the Commission in order to prevent the supply and use of products that pose a risk.
(b)Voluntary measuresU.K.
Under Article 5(3) of the GPSD and Article 22 of Regulation (EC) No 765/2008, economic operators are obliged to notify the competent Member State authorities of voluntary action and measures taken to prevent risks to consumers posed by products they have made available on the market (ideally by means of a ‘Business Gateway’ notification). The authority of a Member State receiving this kind of notification uses this information as the basis for a notification (if all the notification criteria are met) and sends it immediately after receipt of the ‘Business Gateway’ notification.
Where voluntary measures are adopted in the form of an agreement between an economic operator and an authority of a Member State or on the basis of a recommendation from an authority to a producer or distributor, a notification is submitted immediately after the conclusion of such an agreement or the adoption of such a recommendation.
To ensure common application of the notification obligation, Part III, Appendix 4 to these Guidelines lays down specific deadlines for submitting notifications to the Commission via the RAPEX application(32).
3.5.2. Deadlines (33) U.K.
Member States notify the Commission of preventive and restrictive measures adopted as soon as possible and by the deadlines specified in Part III, Appendix 4 to these Guidelines at the latest. Appropriate arrangements are in place at national level concerning the transmission of information between national authorities in charge of product safety and the RAPEX Contact Point to ensure that the deadlines are met. (See Part II, Chapter 5.1).
The deadlines provided apply irrespective of any appeal procedure or official publication requirement.
3.5.3. Emergency situations U.K.
All notifications concerning products posing a serious risk requiring emergency action are preceded by a telephone call from the RAPEX Contact Point to the Commission RAPEX Team's mobile telephone number to facilitate immediate action and follow-up. This rule applies in particular to notifications transmitted at weekends or during holiday periods. (See also Part II, Chapter 5.1).
4. Follow-up activities U.K.
4.1. Communication of follow-up activities U.K.
Member States notify the Commission of any findings subsequent to their follow-up activities in relation to RAPEX notifications (i.e. ‘Article 12 notifications’ and ‘Notifications requiring emergency action’ as well as notifications under Article 22 of Regulation (EC) No 765/2008) and information on products posing a risk sent by the Commission (Chapter 3.3.4).
In addition, Member States are encouraged to notify the Commission of any follow-up activities regarding notifications for less than serious risks and for information.
4.2. Content of follow-up notifications U.K.
4.2.1. Scope of data U.K.
Findings resulting from follow-up activities are communicated to the Commission in the form of follow-up notifications. To harmonise the type of information and to keep the workload to a minimum, Member States submit follow-up notifications in particular in the following situations:
(a)A notified product has been found on the marketU.K.
A follow-up notification is sent when national authorities find the notified product on the market or at the external border. This follow-up notification contains the full details of the product in question (e.g. name, brand, model number, bar code, batch number) plus information on the total number of items found on the market. Furthermore, the following details of the measures taken are communicated: type (compulsory or voluntary), category (e.g. withdrawal from the market, recall from consumers), scope (e.g. country-wide, local), date of entry into force and duration (e.g. permanent, temporary). If the notified product was found on the market but no measures were adopted, specific reasons justifying no measures being taken should be given in the follow-up notification.
To reduce the burden on the national authorities as regards their follow-up practice, Member States do not need to inform the Commission (unless the Commission asks to be informed) of the conclusions of follow-up activities by means of a follow-up notification when the notified product is not found on the market.
(b)Different risk assessmentU.K.
A follow-up notification is sent when the conclusions of a risk assessment performed by an authority of the reacting Member State differ from the conclusions set out in the original notification. This follow-up notification contains a detailed risk description (including the results of tests, a risk assessment and information on known accidents and incidents), accompanied by supporting documents (test reports, certificates, etc.). Furthermore, the reacting Member State should prove that the risk assessment submitted with its follow-up notification was performed on the same product as the one notified, i.e. the same brand, name, model number, batch number, origin, etc.
(c)Additional informationU.K.
A follow-up notification is sent when national authorities collect additional information (during their follow-up activities) that may be useful for market surveillance and enforcement in other Member States.
Member States are encouraged to collect additional information that may be relevant for authorities both in other Member States and in third countries that cooperate closely with the EU on product safety. Details include product origin (e.g. information on the country of origin, manufacturer and/or exporters) and information on the supply chains (e.g. information on the countries of destination, importers and distributors). The country carrying out the follow-up activities attaches all available supporting documents to the follow-up notification, such as copies of orders, sales contracts, invoices, customs declarations, etc.
Member States may also indicate whether certain follow-up actions have been performed although the product has not been found in their territory.
4.2.2. Completeness of follow-up notifications U.K.
The RAPEX Contact Point of the reacting Member State, together with the responsible authority, ensures that all data provided in their follow-up notification is accurate and complete and that there is no confusion with other similar products that are available on the EU market. (See also Part II, Chapter 5.1).
The standard follow-up notification template is provided in Part III, Appendix 2 to these Guidelines. Should certain relevant information not be available when a follow-up notification is submitted, the reacting Member State indicates this on the follow-up form. Once this information becomes available, the reacting Member State may request that its follow-up notification be updated. The updated follow-up notification is examined by the Commission before it is validated and distributed through the system.
The RAPEX Contact Point provides all authorities in its own Member State that participate in the RAPEX network with instructions on the scope of the data required to complete the follow-up notification template correctly. This helps to ensure that information provided by these authorities to the Contact Point is correct and complete. (See Part II, Chapter 5.1).
4.2.3. Updating of validated follow-up notifications U.K.
The reacting Member State informs the Commission (as soon as possible and by the deadlines specified in Part III, Appendix 4 to these Guidelines at the latest) of any developments that may require changes to a follow-up notification distributed through the RAPEX application. In particular, Member States inform the Commission of changes in the status of the measures taken or in the risk assessment submitted with their follow-up notification.
The Commission examines the information provided by the reacting Member State and if necessary updates the information concerned.
4.2.4. Responsibility for follow-up notifications U.K.
Responsibility for the information provided in follow-up notifications lies with the notifying Member State(34).
The authority(ies) involved in the follow-up activities (e.g. by carrying out the risk assessment or by adopting restrictive measures) take responsibility for the information provided in follow-up notifications. The RAPEX Contact Point checks and validates all follow-up notifications prepared by the respective authorities before transmitting them to the Commission. (See also Part II, Chapter 5.1).
Any action taken by the Commission, such as examining and validating follow-up notifications, does not imply any assumption of responsibility for the information transmitted, which remains with the Member State submitting the follow-up notification.
4.2.5. Response to follow-up notifications U.K.
Member States may respond to any follow-up notifications regarding their own notification(s) by starting a discussion on the online collaborative space put at the disposal of Member States for the exchange of information (see Part II Chapter 5.3.2). This ensures that the response is visible to all members of RAPEX.
4.3. Actors and roles involved in follow-up activities U.K.
The parties involved in the follow-up notification process and their responsibilities therein are the following:
4.3.1. Economic operators (35) U.K.
Economic operators are not directly involved in the submission of follow-up notifications. However, economic operators must cooperate with national authorities and provide them with any information concerning a product which is the subject of an existing notification in order to facilitate the creation and submission of follow-up notifications via the RAPEX application.
4.3.2. Market surveillance authorities U.K.
Market surveillance authorities notify the European Commission through the RAPEX application about any follow-up activities or other information regarding notifications.
4.3.3. European Commission U.K.
The European Commission examines and validates follow-up notifications according to the specifications included in Part II, Chapter 4.2.
4.4. Workflow U.K.
4.4.1. Creation and submission of a follow-up notification by a Member State U.K.
The RAPEX Contact Point is responsible for the submission of follow-up notifications via the RAPEX application. (See Part II, Chapter 5.1).
4.4.2. Examination of follow-up notifications by the Commission U.K.
4.4.2.1. Correctness and completeness U.K.
The Commission checks all follow-up notifications received through the RAPEX application before they are validated and transmitted to the Member States. These checks focus on the correctness and completeness of the information provided.
The Commission checks if a follow-up notification meets all the relevant requirements set out in the GPSD and in these Guidelines and if the correct procedure was applied. Once the correctness of a follow-up notification is confirmed, the Commission checks its completeness. Chapter 4.2.2 of these Guidelines is to be used as a point of reference for this examination.
The Commission pays special attention to follow-up notifications containing risk assessments. It verifies, in particular, that the risk description is complete, clearly presented and well documented, and that the risk assessment clearly relates to the product covered by a notification.
4.4.2.2. Requests for additional information U.K.
Before validating a follow-up notification, the Commission may request the reacting Member State to provide additional information or clarification within a given deadline. Validation of a follow-up notification may be conditional upon receipt of the data requested.
The Commission may request the opinion of any Member State and, in particular, the notifying Member State on a validated follow-up notification. The Member State submits its opinion to the Commission within a deadline specified by the latter. Furthermore, the notifying Member State informs the Commission whether any changes to the notification (e.g. to the risk assessment) or to its status (e.g. permanent withdrawal from the system) are necessary.
4.4.3. Validation and distribution of follow-up notifications U.K.
All follow-up notifications assessed as correct and complete are validated and distributed by the Commission according to the deadlines specified in Appendix 5 to these Guidelines.
The Commission does not validate follow-up notifications with a risk assessment different from that of the notification they refer to, if the risk assessment is not complete, clearly presented and well documented, or if it is not shown that the risk assessment was performed in relation to the product covered by the notification.
4.4.4. Permanent withdrawal of a follow-up notification from RAPEX U.K.
Follow-up notifications distributed through the RAPEX application are kept in the system as long as the notification to which they are attached. The Commission may permanently withdraw a validated follow-up notification from the RAPEX application if a notification to which this follow-up notification is attached has been withdrawn from RAPEX (in accordance with Part II, Chapter 3.4.7.1.1 of these Guidelines). Furthermore, the Commission may withdraw a validated follow-up notification where it clearly provides incorrect information, and in particular where:
the product found on the market by the reacting Member State is different from the product covered by the notification;
the measures adopted by the reacting Member State are successfully challenged in court or in other proceedings and subsequently withdrawn;
the risk assessment performed by the reacting Member State is proven to be incorrect or relates to a different product from the one covered by the notification.
The provisions of Chapters 3.4.7.1.2 and 3.4.7.1.3 apply.
Once the Commission decides to withdraw a follow-up notification it is removed from RAPEX (or otherwise made invisible to users of the system).
The Commission informs all Member States of the withdrawal of a follow-up notification via the online collaborative space referred to in Part II, Chapter 5.3.2 or through other equally effective means.
4.5. Deadlines for submitting follow-up notifications U.K.
Member States submit follow-up notifications to the Commission as soon as possible and by the deadlines specified in Appendix 4 to these Guidelines at the latest.
Appropriate arrangements are established at national level concerning the transmission of information between all competent authorities and the RAPEX Contact Point to ensure that the deadlines are met. (See Part II, Chapter 5.1).
The deadlines apply irrespective of any appeal procedure or official publication requirement.
4.6. Requests for confidentiality U.K.
A reacting Member State may request confidentiality in its follow-up notification. Such requests clearly state which part(s) of the follow-up notification should be kept confidential. Furthermore, all requests for confidentiality are accompanied by justification clearly stating the reasons.
Requests for confidentiality are examined by the Commission to determine that they are justified (i.e. in line with the provisions of the GPSD and these Guidelines) and complete (i.e. it states which parts of the form that it covers and if it contains a justification). The final decision on confidentiality is taken by the Commission after consultation of the responsible RAPEX Contact Point. (See Part II, Chapter 5.1).
The Commission and the Member States treat follow-up notifications with requests for confidentiality in the same way as the other follow-up notifications. The confidentiality of a follow-up notification or parts of it does not prevent it from being distributed through the RAPEX application to the competent national authorities. However, neither the Commission nor the Member States should disclose any parts of a follow-up notification that are confidential to the public. This information is confidential and therefore cannot be published in any form.
The Member State submitting the follow-up notification withdraws its request for confidentiality immediately after it becomes aware that the reasons for such a request are no longer valid. The Commission informs all Member States of the withdrawal of the confidentiality after the receipt of such a request from the reacting Member State.
5. RAPEX networks U.K.
5.1. RAPEX National Contact Points U.K.
Each Member State establishes a single RAPEX Contact Point to operate RAPEX at national level. The Member States decide within which national authority to set up the RAPEX contact point. Each Member State also organises its national RAPEX network to ensure the efficient flow of information between the national contact point and the various authorities participating in RAPEX. (See Part I Chapter 5.4 and Part II, Chapter 1.2).
5.1.1. Organisation U.K.
Each Member State gives the national Contact Point the resources and information it needs to perform its tasks and, in particular, to operate the system with effective back-up/business continuity.
The RAPEX Contact Point has a separate email account for RAPEX, accessible to all officials in that contact point (e.g. rapex@ …). Professional or private email accounts of officials in charge of the RAPEX Contact Point should not be used as the email account of the RAPEX Contact Point. The RAPEX Contact Point also has a direct phone number through which it can be reached during and outside working hours.
5.1.2. Tasks U.K.
The main tasks of the RAPEX Contact Point are to:
organise and steer the work of the national RAPEX network, in accordance with the rules set out in these Guidelines;
train and assist all authorities in the network in the use of RAPEX;
ensure that all RAPEX tasks stemming from the GPSD and these Guidelines are performed correctly and, in particular, that all required information (i.e. notifications, follow-up notifications, additional information, etc.) is provided to the Commission without delay;
transmit information between the Commission and the national market surveillance authorities and authorities in charge of external border controls;
check and validate the completeness of the information received from all authorities before transmission to the Commission through the RAPEX application;
check before submitting a notification whether a product has already been notified or information on that product has been exchanged through the RAPEX application (to avoid any duplication);
participate in RAPEX Contact Point Working Group meetings and other events on the operation of RAPEX;
suggest possible improvements to the operation of the system;
inform the Commission immediately of any technical problems with the functioning of the RAPEX application;
coordinate all national activities and initiatives carried out in relation to RAPEX;
explain to stakeholders how RAPEX operates and clarify their obligations, particularly for the business notification obligation set out in Article 5(3) of the GPSD.
5.2. RAPEX networks established at EU and national levels U.K.
5.2.1. The RAPEX Contact Point Network U.K.
The Commission organises and steers the work of the RAPEX Contact Point Network. This network consists of all RAPEX Contact Points appointed in the Member States and European Economic Area (EEA) countries.
The Commission regularly convenes meetings of the RAPEX Contact Point Network to discuss the operation of the system (e.g. to communicate the latest developments concerning RAPEX, to exchange experience and ‘know-how’), and to improve cooperation between the RAPEX Contact Points.
5.2.2. RAPEX networks established at national level U.K.
The RAPEX Contact Points organise and steer the work of their own ‘RAPEX national network’. The network consists of:
the RAPEX Contact Point;
market surveillance authorities responsible for monitoring the safety of products; and
authorities in charge of external border controls.
RAPEX Contact Points are encouraged to provide for the organisation and operation of the RAPEX national network so as to ensure that all the authorities involved are aware of their roles and responsibilities as regards the operation of RAPEX. This should be consistent with the information contained in these Guidelines.
The RAPEX Contact Points are encouraged to facilitate regular and continuous exchange of information and discussion with their national network in order to discuss with all the authorities involved how RAPEX is organised, how it operates and, if necessary, to give training courses.
5.3. RAPEX internal communication tools, practical and technical arrangements for RAPEX and best practice U.K.
5.3.1. Languages U.K.
The use of languages in notifications and follow-up notifications, as well as communications between the RAPEX Contact Points and the Commission, must take account of the objectives of RAPEX and must ensure a rapid exchange of information between Member States and the Commission on products posing serious risks.
To facilitate the work of the network, Member States authorities are encouraged to use the existing EC eTranslation webpage to ensure all Member States understand what is being communicated through RAPEX.
A link to this translation tool to submit documents or extracts from texts for translation from and into all EU languages(36) is available in the collaborative space. (See Part II, Chapter 5.3.2).
5.3.2. RAPEX online tools U.K.
(a)RAPEX systemU.K.
The Commission has established and maintains a web-based application for use as a communication tool for the purpose of RAPEX. Member States use this system to create and submit notifications and follow-up notifications through the RAPEX application, and the Commission uses it to validate and distribute the documents it receives.
The Commission provides access to the system to all RAPEX Contact Points, competent national authorities and the relevant Commission departments. The Commission lays down the rules for granting access to the system and gives access to as many users as possible, taking into account needs and technical limitations.
Where the RAPEX system is temporarily not operational (for reasons other than regular and planned maintenance work), Member States should only submit notifications of serious risks to the Commission (i.e. ‘Article 12 notifications’, ‘Article 12 notifications requiring emergency action’ or ‘Article 22 of Regulation (EC) No 765/2008) notifications’.
The submission of other notifications and follow-up notifications is suspended until the RAPEX system is re-established. While the system is not operational, RAPEX notifications should be sent to the Commission by email to: just-rapex@ec.europa.eu or to another email address communicated in advance. If email transmission is not possible, RAPEX notifications are sent to the Commission by any other means considered appropriate(37).
(b)‘Product Safety Business Alert Gateway’U.K.
The ‘Product Safety Business Alert Gateway’ (also known as the ‘Business Gateway’) is intended to simplify the practical aspects of the obligation on producers and distributors, or their authorised representative, under Article 5(3) of the GPSD to notify the competent national authorities of the Member States if they know or ought to know, on the basis of the information in their possession and as professionals, that a product they have placed on the market is dangerous.
The ‘Business Gateway’ consists of two elements: (i) the notification template and (ii) the online database. The notification template is reserved for use by producers and distributors to inform the competent national authorities of the Member States that a product they have placed on the market is dangerous, in line with their obligation under Article 5(3) of the GPSD. The online database is intended for use by Member States national authorities responsible for receiving notifications of dangerous consumer products submitted by producers and distributors. The competent national authority may use the information provided to submit a RAPEX notification if all criteria for this are met.
(c)Collaborative spaceU.K.
The Commission also manages a collaborative space to exchange information between the Commission and the Member States competent national authorities. This includes the EU Consumer Product Safety platform, open to the RAPEX Contact Points and their colleagues working on product safety issues in the competent national authorities for all RAPEX-related issues. Requests for access to the space must be made by the RAPEX Contact Points in the relevant Member State and authorised by the Commission.
This space also includes a section, managed by the Commission, containing useful tips and information on the functioning of RAPEX and input from the Member States.
(d)‘RAG tool’(38) U.K.
The Commission has developed this tool available on the RAPEX website to facilitate the risk assessment of products notified through the RAPEX system, in accordance with the principles laid down in Appendix 6.
5.3.3. Contact details U.K.
The Commission provides the RAPEX Contact Points with the contact details of the Commission's RAPEX team, including names, email addresses and telephone numbers.
The RAPEX Contact Points provide the Commission with their contact details, including the names of officials working within the Contact Point, the name and address of the authority where the RAPEX Contact Point is established, the email addresses and phone numbers of officials. Any changes to the contact details are immediately communicated to the Commission by the RAPEX Contact Point. The Commission publishes and updates a list of contact details of the RAPEX Contact Points on the RAPEX website.
Member States process contact details including personal data in application of the EU's data protection legislation. On the information exchange through RAPEX, Member States should process personal data ensuring that it circulates and is distributed only as far as it is strictly necessary.
5.3.4. Operation of RAPEX outside regular working hours U.K.
RAPEX operates non-stop. The Commission and the RAPEX Contact Points ensure that officials responsible for operating RAPEX can always be contacted (by phone, e-mail or other equally effective means) and that they can take whatever action is necessary, including in an emergency and outside regular working hours, such as weekends and holidays.
The Commission provides the RAPEX Contact Points with an emergency telephone number, which should be used to contact the Commission RAPEX team outside of working hours, with priority over any other communication channels.
The RAPEX Contact Points provide the Commission with their contact details, including the phone numbers of officials who can be contacted during and outside working hours. Any changes to the contact details are immediately communicated to the Commission by the RAPEX Contact Points.
PART III U.K.APPENDICES
1. Fields and information included in notifications (39) U.K.
Fields that will be published on the web are shaded.
| * Indicates a mandatory field. | |
| Notification Form | |
|---|---|
| Section 1: General information | |
| Case number | |
| Creation date | |
| Validation/distribution date | |
| Notification type * | |
| Notifying country | |
| Full Contact details of the Notifying Authority * | |
| Section 2: Product | |
| Professional / Consumer Product | |
| Product category * | |
| OECD Portal category (if known) | |
| Product (what the product is) * | |
| Name * | |
| Brand * | |
| Type/number of model: * | |
| Batch number/Bar code * | |
| Customs code * | |
| Product and packaging description * | |
| Total number of items covered by the notification (if known) * | |
| Photos: | |
| Section 3: Regulations and standards applicable | |
| Legal provisions (directive, decision, regulation, etc.) * | |
| Standards * | |
| Proof of conformity * | |
| Is the product counterfeit? * | |
| Certificates | |
| Section 4: Traceability | |
| Country of origin (where the product manufactured) * | |
| Countries of destination * | |
| Full Contact details of the manufacturer or its representative(s) * | |
| Full Contact details of the exporter(s) * | |
| Full Contact details of the importer(s) * | |
| Full Contact details of the distributor(s) * | |
| Full Contact details of the retailer(s) * | |
| Is the product (also) sold online? | |
| Please give details: URL | |
| Section 5: Risk assessment | |
| Risk category * | |
| Risk level | |
| Summary of test results * | |
| Description of the technical issue that leads to the highest risk level | |
| Risk description (how the technical defect leads to the risk) * | |
| EU Legal provisions and /or Standards against which the product was tested and did not comply with * | |
| Information on known incidents and accidents * | |
| Section 6: Measures | |
| Type of measures adopted * | |
| If Voluntary: | Type of economic operator taking notified measure(s) * |
| Name of economic operator taking notified measure(s) * | |
| If Compulsory: | Name of authority ordering the notified measure(s) * |
| Type of economic operator to whom the measure(s) were ordered * | |
| Category of measures * | |
| Date of entry into force * | |
| Duration * | |
| Scope * | |
| Has the notification been sent by a producer or a distributor under Article 5(3) of the GPSD? * | |
| URL link to company recall page (if available) | |
| Section 7: Confidentiality | |
| Is the notification confidential? * | |
| Scope of confidentiality | |
| Justification | |
| Section 8: Other | |
| Additional information | |
| Justification for sending ‘Notification for information’ | |
| Annexes | |
| Photos (products, packaging and label) | |
| Certificates | |
| Test report and risk assessment | |
| Notification sent by an economic operator through ‘Business Gateway’ | |
| Adopted measures | |
2. Fields and information included in follow-up notifications (40) U.K.
Fields that will be published on the web are shaded.
| * Indicates a mandatory field | |
| Section 1: General information | |
|---|---|
| Case number | |
| Validated notification type | |
| Notifying country | |
| Creation date | |
| Validation/distribution date | |
| Submission number | |
| Follow up notification number | |
| Reacting country | |
| Full contact details of the notifying authority | |
| Validated notification product category | |
| Notified product | |
| Notified name | |
| Product (what the product is) | |
| Name (on the product or the packaging) | |
| Brand (on the product or the packaging) | |
| Type/number of model | |
| Batch number/Bar code (or other information to identify which products are affected) | |
| Photos (products, packaging and label) | |
| Section 2: Type of follow-up notification | |
| Product found * | |
| Total number of items found (if known) * | |
| Measures adopted / Measures not adopted | |
| Type of measures adopted * | |
| If Voluntary: | Type of economic operator taking notified measure(s) * |
| Name of economic operator taking notified measure(s) * | |
| If Compulsory: | Name of authority ordering the notified measure(s) * |
| Type of economic operator to whom the measure(s) were ordered * | |
| Category of measures * | |
| Date of entry into force * | |
| Duration * | |
| Scope * | |
| Adopted measures | |
| URL link to company recall page (if available): | |
| Different risk assessment * | |
| Risk category * | |
| Summary of the test results (description of technical defects) * | |
| Indication of legal provisions and standards (with clauses) against which the product was tested * | |
| Different risk assessment * | |
| Information on known incidents and accidents * | |
| Attachments (certificates, test report and risk assessment …) | |
| Additional information * | |
| Complementary information on distribution channels and/or product's origin | |
| Complementary information on the risk assessment | |
| Other complementary information | |
| Section 3: Confidentiality | |
| Is the follow-up confidential? * | |
| Scope of confidentiality | |
| Justification | |
| Annexes | |
| Photos (product, packaging and label) | |
| Test reports and risk assessments | |
| Certificates | |
| Adopted measures | |
3. Notification scheme U.K.
4. Deadlines for member states U.K.
Member States are required to act within the deadlines indicated unless duly justified
| Notification procedure | Action | Deadline | ||
|---|---|---|---|---|
| Notifications | Send ‘Article 12 notification requiring emergency action’ | Within 3 days after:
| ||
| Send ‘Article 12 notification’ or Article 22 Regulation (EC) No 765/2008 notification | Within 10 days after:
| |||
| Confirm measures if the notification was sent before deciding to adopt measures | Within 45 days after submission of the notification | |||
| Update to a notification | Within 5 days after receipt of the information on developments requiring changes to a notification | |||
| Follow-up notifications | Ensure follow-up activities to: | ‘Article 12 notification requiring emergency action’ | Within 20 days after receipt of a notification | |
| ‘Article 12 notification’ and to ‘Notification sent by the European Commission’ as well as Article 22 of Regulation (EC) No 765/2008 notification | Within 45 days after receipt of a notification | |||
| Send follow-up notification to: | ‘Article 12 notification requiring emergency action’ | Within 3 days after:
| ||
| ‘Article 12 notification’ and to ‘Notification sent by the European Commission’ as well as Article 22 of Regulation (EC) No 765/2008 notification | Within 5 days after:
| |||
| Update to a follow-up notification | Within 5 days after receipt of information or developments requiring changes to a follow-up notification | |||
| Notification procedure established under Article 11 of the GPSD | Notifications | Send ‘Article 11 notification’ | Within 10 days after adoption of ‘Compulsory measures’ | |
| Update to the notification | Within 5 days after receipt of information on developments requiring changes to the notification | |||
5. Deadlines for the Commission U.K.
| Notification procedure | Action | Deadline | |
|---|---|---|---|
| EU Rapid Information System ‘RAPEX’ established under Article 12 of the GPSD | Notifications | Validate ‘Article 12 notification requiring emergency action’ | Within 3 days after receipt of a notification |
| Validate ‘Article 12 notification’ as well as Article 22 of Regulation (EC) No 765/2008 notification | Within 5 days after receipt of a notification | ||
| Validate ‘Notification for information’ | Within 10 days after receipt of a notification | ||
| Follow-up notifications | Validate follow-up notification sent to ‘Article 12 notification requiring emergency action’ | Within 3 days after receipt of a follow-up notification | |
| Validate follow-up notification sent to ‘Article 12 notification’ and to ‘Notification sent by the European Commission’ as well as Article 22 of Regulation (EC) No 765/2008 notification | Within 5 days after receipt of a follow-up notification | ||
| Validate follow-up notification sent to ‘Notification for information’ | Within 10 days after receipt of a follow-up notification | ||
| Notification procedure established under Article 11 of the GPSD | Notifications | Validate ‘Article 11 notification’ | Within 10 days after receipt of a notification |
| Follow-up notifications | Validate follow-up notifications sent to ‘Article 11 notification’ | Within 10 days after receipt of a follow-up notification | |
In other places of these Guidelines, the term ‘Commission’ generally refers to the RAPEX team established in the Commission department responsible for Directive 2001/95/EC and to the relevant Commission services, where appropriate.
The Information and Communication System on Market Surveillance (‘ICSMS’). This platform is aimed at facilitating communication between market surveillance bodies in the EU and in EFTA countries on non-compliant products.
In the context of this document, the term ‘Member States’ must be interpreted as not precluding all other actors from being addressed by the provisions contained in these Guidelines.
See the latest EC Implementing Decision published on https://ec.europa.eu/consumers/consumers_safety/safety_products/rapex/alerts/repository/content/pages/rapex/index_en.htm
See recital 10 of Directive 2001/95/EC.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety (OJ L 31, 1.2.2002, p. 1).
Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use (OJ L 311, 28.11.2001, p. 67).
Directive 2001/82/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to veterinary medicinal products (OJ L 311, 28.11.2001, p. 1).
Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices, amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and repealing Council Directives 90/385/EEC and 93/42/EEC (OJ L 117, 5.5.2017, p. 1).
Council Directive 90/385/EEC of 20 June 1990 on the approximation of the laws of the Member States relating to active implantable medical devices (OJ L 189, 20.7.1990, p. 17).
Directive (EU) 2015/1535 of the European Parliament and of the Council of 9 September 2015 laying down a procedure for the provision of information in the field of technical regulations and of rules on Information Society services (OJ L 241, 17.9.2015, p. 1).
See EU general risk assessment methodology (Action 5 of Multi-Annual Action Plan for the surveillance of products in the EU (COM(2013)76) providing guidance to authorities with relation to Article 20(2) of Regulation (EC) No 765/2008: http://ec.europa.eu/DocsRoom/documents/17107/attachments/1/translations
See https://ec.europa.eu/consumers/consumer-safety/rag/#/screen/home
www.ec.europa.eu/rapex
See Part I, Chapter 6.2 of these Guidelines.
See Part II, Chapter 2.2.1 of these Guidelines.
See Part II, Chapter 2.2.1 of these Guidelines.
See Part II, Chapter 2.2.2 of these Guidelines.
See Part II, Chapter 2.2.2 of these Guidelines.
For more information on follow-up actions, see Part II Chapter 4.4.5 of these Guidelines.
For more information about notifications where safety aspects are subject to discussions at EU level, see Part II Chapters 3.4.4 and 3.4.7.1.1 of these Guidelines.
See point 10 of Annex II of Directive 2001/95/EC.
See point 9 of Annex II of Directive 2001/95/EC.
For more information on notifications on safety aspects subject to discussions at EU level, see Part II Chapters 3.1.2(d) and 3.4.7.1.1.
https://ec.europa.eu/consumers/consumers_safety/safety_products/rapex/alerts/?event=main.search
Practice already agreed at the GPSD Committee of 24 September 2012, of which RAPEX Contact Points were informed at the RAPEX Contact Point meeting of 4 October (agenda point 4) and applied since 2013.
Paragraph 1 of Article 16(1) of Directive 2001/95/EC and Article 23 paragraph 3 in relation to Article 19(5) of Regulation (EC) No 765/2008.
Article 16(1) and (2) of Directive 2001/95/EC.
For more information on the notification criteria, see Part I Chapter 2.
For more information on notifications sent through the RAPEX application before measures are taken, see Chapter 3.1.2(b).
For more information on notifications on safety aspects subject to discussions at EU level, see Part II Chapters 3.1.2.d and 3.4.4.
For more information about deadlines, see Part III Appendix 4 of these Guidelines.
All deadlines mentioned in these Guidelines are expressed in calendar days.
See point 10 of Annex II of Directive 2001/95/EC.
For the purpose of these Guidelines, ‘economic operator’ refers to any natural of legal person defined as ‘economic operator’ in Regulation (EC) No 765/2008 or as ‘producer’ and ‘distributor’ in the GPSD.
https://webgate.ec.europa.eu/etranslation/translateDocument.html
There is no need to send notifications via the Permanent Representation of a Member State to the EU.
See Part I, Chapter 5.3 of these Guidelines.
The fields contained in the template may be updated following developments agreed between the Commission and Member States.
The fields contained in the template may be updated following developments agreed between the Commission and Member States.