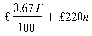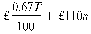- Latest available (Revised)
- Original (As made)
The Veterinary Medicines Regulations 2006
You are here:
More Resources
Status:
This is the original version (as it was originally made).
PART 2Fees relating to marketing authorisations
Fees for specified pharmaceutical applications
6. The following table sets out the fees relating to a pharmaceutical veterinary medicinal product for—
(a)a national application for a marketing authorisation that is—
(i)a full application under Part 1 of Schedule 1;
(ii)a bibliographic application; or
(iii)an application based on pharmacological equivalence;
(b)an application for a marketing authorisation using the decentralised procedure where the United Kingdom is a concerned member State;
(c)an application for the mutual recognition of a product authorised in another member State.
Fees for specified pharmaceutical applications
| Pharmacologically equivalent national application | |||||
|---|---|---|---|---|---|
| Menu | Full national application under Part 1 of Schedule 1 (£) | Bibliographic national application (£) | Reference product authorised in UK (£) | Reference product not authorised in UK (£) | Decentralised application where the UK is a concerned member State or recognition of a product authorised in another member State (£) |
| Base Fee: The following fees are in addition to the base fee— | 910 | 1,800 | 1,800 | 2,320 | 460 |
| Quality assessment (if quality data are assessed): | 3,810 | 3,230 | 2,710 | 3,470 | 1,810 |
| Safety assessment (if safety data are assessed): | 3,810 | 3,030 | 1,030 | 1,330 | 1,810 |
| Efficacy assessment (if efficacy data are assessed): | 3,810 | 3,030 | 1,030 | 1,330 | 1,810 |
| Ecotoxicology assessment (if ecotoxicology data are assessed): | 640 | 520 | 320 | 410 | 390 |
| Additional fee if any of the target species is a food-producing animal (not payable if neither safety data nor ecotoxicology data are assessed): | 3,740 | 3,420 | 2,070 | 2,650 | 1,350 |
| Reduced by— | |||||
| 2,100 | 2,100 | 1,290 | 1,650 | 640 |
| 990 | 760 | 290 | 370 | 290 |
| Additional fee for each active ingredient not previously included in a veterinary medicinal product authorised in the United Kingdom— | |||||
| 7,170 | 6,330 | 5,610 | 7,200 | 2,520 |
| 6,260 | 5,620 | 5,360 | 6,870 | 2,200 |
| Additional fee for each additional pack type: | 720 | 720 | 590 | 740 | 330 |
| Reduced by— | |||||
| 350 | 350 | 350 | 450 | 120 |
| 180 | 180 | 120 | 150 | 60 |
| 60 | 60 | 60 | 70 | 60 |
| 60 | 60 | — | — | 60 |
| Additional fee for each additional active ingredient (food-producing animal): | 6,210 | 5,870 | 3,880 | 4,960 | 2,000 |
| Reduced by— | |||||
| 1,400 | 1,400 | 1,400 | 1,790 | 470 |
| 2,630 | 2,630 | 1,580 | 2,020 | 820 |
| 880 | 700 | 530 | 670 | 290 |
| 700 | 580 | — | — | 230 |
| Additional fee for each additional active ingredient (non-food-producing animal): | 4,140 | 3,940 | 3,110 | 3,960 | 1,430 |
| Reduced by— | |||||
| 1,400 | 1,400 | 1,400 | 1,790 | 470 |
| 1,400 | 1,400 | 880 | 1,120 | 470 |
| 880 | 700 | 530 | 670 | 290 |
| 60 | 60 | — | — | 60 |
| Additional fee if there is more than one target species, for each additional species (food-producing animal): | 3,820 | 3,430 | 2,330 | 2,980 | 1,240 |
| Reduced by— | |||||
| 180 | 180 | 180 | 220 | 60 |
| 1,400 | 1,400 | 880 | 1,120 | 470 |
| 1,750 | 1,400 | 1,050 | 1,350 | 530 |
| 120 | 120 | — | — | 60 |
| Additional fee if there is more than one target species, for each additional species (non-food-producing animal): | 2,400 | 2,010 | 1,490 | 1,900 | 780 |
| Reduced by— | |||||
| 180 | 180 | 180 | 220 | 60 |
| 180 | 180 | 120 | 150 | 60 |
| 1,750 | 1,400 | 1,050 | 1,350 | 530 |
| 60 | 60 | — | — | 60 |
| Additional fee for each additional recommended route of administration (food-producing animal): | 2,590 | 2,390 | 1,560 | 1,980 | 910 |
| Reduced by— | |||||
| 1,400 | 1,400 | 880 | 1,120 | 470 |
| 880 | 700 | 530 | 670 | 290 |
| 60 | 60 | — | — | 60 |
| Additional fee for each additional recommended route of administration (non- food-producing animal): | 1,170 | 970 | 720 | 910 | 390 |
| Reduced by— | |||||
| 180 | 180 | 120 | 150 | 60 |
| 880 | 700 | 530 | 670 | 290 |
| Simultaneous applications: fee for each additional product in the application: | 2,780 | 2,780 | 2,780 | 3,560 | 1,610 |
Decentralised pharmaceutical application where the United Kingdom is the reference member State
7.—(1) The fee for a decentralised application for a pharmaceutical product where the United Kingdom is the reference member State is the same as for a national application as set out in the table in paragraph 6, with the addition of the fees in the following table.
Decentralised pharmaceutical application where the United Kingdom is the reference member State
| Application | Additional fee (£) |
|---|---|
| Food-producing animal: one concerned member State: | 3,560 |
| Non-food-producing animal: one concerned member State: | 3,100 |
| Each additional concerned member State: | 510 |
(2) In the case of a simultaneous application, the fee for each additional product in the application is £6,400 for one concerned member State and £110 for each additional concerned member State.
Application for a marketing authorisation for an immunological product
8.—(1) The fee for a national application for a marketing authorisation relating to an immunological product, a decentralised application where the United Kingdom is the concerned member State or the mutual recognition of a product authorised in another member State is in accordance with the following table.
Fees for specified immunological applications
| Menu | National application for a marketing authorisation (£) | Decentralised application where the UK is a concerned member State or recognition of a product authorised in another member State (£) |
|---|---|---|
| Base fee: | 11,310 | 5,560 |
The following fees are in addition to the base fee— Additional fee for each active ingredient not previously included in a veterinary medicinal product authorised in the United Kingdom, and for each new combination of active ingredients: | 7,100 | 2,390 |
| Additional fee for each adjuvant or preservative not previously included in a veterinary medicinal product authorised in the United Kingdom and for each new combination of adjuvants or preservatives: | 1,300 | 640 |
| More than one antigenic component—fee for each additional component: | 1,290 | 390 |
| More than one species—fee for each additional species: | 5,170 | 1,550 |
| More than one route of administration—fee for each additional route of administration: | 5,170 | 1,550 |
| Simultaneous application—fee for each additional product in the application: | 2,780 | 1,610 |
(2) The fee for an application for a marketing authorisation for an immunological product that is identical to a product already authorised in the United Kingdom but with a lesser number of antigens and which only contains antigens contained in the product already authorised is £10,020 (United Kingdom only) or £5,170 (decentralised application where the United Kingdom is a concerned member State).
Decentralised immunological application where the United Kingdom is the reference member State
9.—(1) The fee for a decentralised application for a marketing authorisation for an immunological product where the United Kingdom is the reference member State is the same as for a national application as set out in the table in paragraph 8(1), with the additions of £3,330 for one concerned member State and £510 for each additional concerned member State.
(2) In the case of a simultaneous application the fee for each additional product in the application is £6,400 for one concerned member State and £110 for each additional concerned member State.
Application for a marketing authorisation using identical data
10. The fee for an application for a marketing authorisation using identical data is in accordance with the following table.
Identical data
| Application | Fee (£) |
|---|---|
| Any application other than decentralised where the United Kingdom is the reference member State: | 910 |
| Decentralised application where the United Kingdom is the reference member State— | |
| 4,000 |
| 510 |
Application for a provisional marketing authorisation
11. The fee for an application for a provisional marketing authorisation is the same as that for a full national marketing authorisation in paragraph 6 (in the case of a pharmaceutical product) or the fee for a national application in paragraph 8 (in the case of an immunological product), and the fee for its conversion into a full marketing authorisation is—
(a)if the application for the full marketing authorisation is received within two years of the grant of the provisional marketing authorisation—
(i)£8,130, or
(ii)if the application for the provisional marketing authorisation was made before 1st October 2006, £10,705; and
(b)in any other case the same fee as for the provisional marketing authorisation.
Application for a marketing authorisation relating to a parallel import
12. The fee for a marketing authorisation for a parallel import is in accordance with the following table.
Parallel imports
| Application | Fee (£) |
|---|---|
| Application where the imported product has been authorised in accordance with the mutual recognition procedure or decentralised procedure, and the United Kingdom is included in these procedures— | |
| 1,690 |
| 340 |
| Any other application—fee for each member State from which the product is imported: | 2,050 |
Application for a variation
13.—(1) An applicant must make a separate application for a variation for each change in the marketing authorisation (unless a change is a direct consequence of the first change) and the appropriate fee is payable for each application.
(2) As an exception from sub-paragraph (1), if an applicant applies for more than one variation to the quality data in a marketing authorisation on the same application form, he may elect to pay a total fee of £4,440; but this sub-paragraph does not apply—
(a)if one or more of the variations relates to a new source of an active substance and the applicant does not submit a Certificate of Suitability issued by the European Pharmacopeia relating to the new source, or
(b)if a significant formulation change is applied for that requires a new assessment of the safety or efficacy of the veterinary medicinal product.
(3) If the variation is one specified in Annex I to Commission Regulation (EC) No. 1084/2003, the fee is £440 for a variation specified as Type 1A in that Annex.
(4) If the variation is specified as Type 1B in that Annex, the fee is £835 except in accordance with the following table.
Reductions to Type 1B fees
| Variation | Conditions | Fee (£) |
|---|---|---|
| Identical changes to a number of products— | All the products are from the same marketing authorisation holder Supporting data are identical All applications are submitted at the same time | First product 835 Each subsequent product 440 |
(5) The fee for a variation classified as Type II in Article 3 of Commission Regulation (EC) No.1084/2003 is £2,220 except in the following cases, where the fee is as specified.
Reductions to Type II fees
| Change | Conditions | Fee (£) |
|---|---|---|
(a)Identical changes to a number of products— | All the products are from the same marketing authorisation holder Supporting data are identical All applications are submitted at the same time | First product2,220 Each subsequent product440 |
(b)Change of distributor— | No other aspect of the dossier is changed and the marketing authorisation holder remains the same | 835 |
(c)Change of legal entity of marketing authorisation holder— | No other aspect of the dossier is changed | 835 |
(d)Simple dosage instruction changes intended to remove ambiguity— | The change is not as a result of safety concerns No new studies are required to support the change The dosage regime remains the same | 835 |
(e)Addition or change to safety warnings— | No other aspects of the dossier are changed No safety warnings are removed No new studies are required to support the change and the proposed warnings serve to increase the protection of the user/environment/target species as appropriate | 835 |
(f)Corrections or simple text layout changes to summary of product characteristics and/or product literature. Included in this is the introduction of multilingual labelling— | The changes are not a result of safety concerns No new studies are required to support the change and no other aspect of the dossier is changed The legibility of the current English labelling is not compromised The indications and warnings are the same in all languages | 835 |
(g)Abbreviated resubmission of a previously refused Type II variation— | At the time of refusal of a Type II variation, the Secretary of State has given written permission for resubmission under this category The application has been resubmitted within 3 months of the date the refusal advice was issued | 835 |
(h)Submission made following the formal advice of the Secretary of State— | The Secretary of State has already assessed the relevant data and formed an opinion on these The change is not required as a result of the holder failing to keep the Part II (quality) data in accordance with current practice or in line with current guidelines issued by the Committee for Medicinal Products for Veterinary Use(1) | 835 |
(i)Approval of a mock-up for an authorised pack size— | The pack size is already authorised No new studies are required to support the change and no other aspect of the dossier is changed | 835 |
(j)Changes to the summary of product characteristics and product literature of a Marketing Authorisation for Parallel Import as a direct consequence of the approval of a variation to the summary of product characteristics and product literature for the United Kingdom authorised product— | The only changes to the summary of product characteristics and product literature are those required to bring the marketing authorisation for parallel import back in direct line with those of the United Kingdom authorised product | 835 |
Application for a variation to a marketing authorisation that has been issued in other member States
14.—(1) In this paragraph the types of variation are those specified in Commission Regulation (EC) 1084/2003.
(2) An applicant must make a separate application for a variation for each change in the marketing authorisation (unless a change is a direct consequence of the first change).
(3) The fee is in accordance with the following table.
Variations
| Type of variation | UK is the reference member State (£) | UK is a concerned member State (£) |
|---|---|---|
| Type II variation: | 8,990 | 2,220 |
If a marketing authorisation holder applies for a Type II variation for a number of marketing authorisations, and—
| ||
| 8,990 | 2,220 |
| 1,555 | 440 |
If a marketing authorisation holder—
| 2,455 | 475 |
| Type 1A variation: | 1,555 | 440 |
| Type 1B variation: | 2,455 | 475 |
If a marketing authorisation holder applies for a Type 1B variation for a number of marketing authorisations, and—
the fee payable is— | ||
| 2,455 | 475 |
| 1,555 | 440 |
Application for an extension to a marketing authorisation
15. The fee for an application for an extension to a marketing authorisation is in accordance with the following table.
Extension to a marketing authorisation
| Extension | Fee if the marketing authorisation is UK only (£) | Fee for a decentralised application where the United Kingdom is a concerned member State or the mutual recognition of an extension authorised in another member State (£) |
|---|---|---|
| Change of strength or potency or the addition of a new strength or potency: | 6,400 | 3,180 |
| Change of pharmaceutical form or the addition of a new pharmaceutical form: | 8,080 | 3,690 |
Change of route of administration, or the addition of a new one, of—
| 5,170 | 2,790 |
| 6,850 | 3,300 |
| Change or addition of target species: | 9,240 | 4,080 |
| Change of active substance: | 8,080 | 3,690 |
| Other: | 8,080 | 3,690 |
| Simultaneous application —fee for each additional product in the application: | 2,780 | 1,610 |
Decentralised application for an extension where the United Kingdom is the reference member State
16.—(1) The fee for a decentralised application for an extension where the United Kingdom is the reference member State is the same as for a national application as set out in the table in paragraph 15, with the additions of the fees in the following table.
Decentralised application for an extension where the United Kingdom is the reference member State
| Application | Additional fee (£) |
|---|---|
| Pharmaceutical product for a food-producing animal—one concerned member State: | 3,560 |
| Pharmaceutical product for a non-food-producing animal—one concerned member State: | 3,100 |
| Immunological product—one concerned member State: | 3,300 |
| Each additional concerned member State: | 510 |
(2) In the case of a simultaneous application, the fee for each additional product in the application is £6,400 for one concerned member State and £110 for each additional concerned member State.
Provision of information relating to the recognition of a United Kingdom marketing authorisation
17.—(1) Where an application is made for the Secretary of State to provide information to other member States to enable them to recognise a marketing authorisation already granted by the United Kingdom the following fees are payable.
(2) Where a valid application to provide information to another member State is received within six months of the original grant of the marketing authorisation, or where the Secretary of State has already provided the information to a member State, and a further valid application is made for him to provide the information to an additional member State within six months of the date he last provided the information the fees are—
| Type of application | Fee (£) |
|---|---|
| Pharmaceutical product for a food-producing animal—one member State: | 2,345 |
| Pharmaceutical product for a non-food-producing animal—one member State: | 1,820 |
| Immunological product—one member State: | 2,050 |
| Each additional member State: | 510 |
(3) In any other case the fees are—
| Type of application | Fee (£) |
|---|---|
| Pharmaceutical product for a food-producing animal—one member State: | 10,105 |
| Pharmaceutical product for a non-food-producing animal—one member State: | 7,080 |
| Immunological product—one member State: | 8,595 |
| Each additional member State: | 510 |
(4) In the case of simultaneous applications, the above fees are payable for each additional product in the application for one member State, with a fee of £110 for each additional product for each additional member State.
Application for the renewal of a national marketing authorisation
18.—(1) The fee for the renewal of a national marketing authorisation originally granted on or after 30th October 2005 is £1,305.
(2) In the case of a marketing authorisation originally granted before 30th October 2005—
(a)if it is the first time the marketing authorisation has been renewed, or if the renewal entails assessment of post authorisation commitments the fee is £1,305, and
(b)otherwise £295.
(3) The fee for the first reassessment of a provisional marketing authorisation is £295, and the fee for each subsequent reassessment is £1,305.
Application for the renewal of a marketing authorisation granted in more than one member State
19. The fee for the renewal of a marketing authorisation granted in more than one member State is—
(a)£1,765 if the United Kingdom is the reference member State, and
(b)£1,175 where the United Kingdom is a concerned member State.
Registration of a homeopathic remedy
20. The fee for an application for the registration of a homeopathic remedy is in accordance with the following table.
Fee for the registration of a homeopathic remedy
| Type of application | Fee (£) |
|---|---|
If all stocks and the formulation have already been assessed by the Secretary of State—
| 155 |
| 360 |
If either all the stocks have already been assessed by the Secretary of State but there is a new formulation, or the formulation has already been assessed by the Secretary of State but one or more of the stocks have not been already assessed—
| 440 |
| 640 |
If the formulation and at least one of the stocks has not already been assessed by the Secretary of State—
| 730 |
| 945 |
If the product is already authorised for human use in the United Kingdom, or for human or veterinary use in the United Kingdom or in another member State—
| 155 |
| 360 |
Annual fees for marketing authorisations
21.—(1) Within 30 days of receiving a written demand from the Secretary of State, a holder of a marketing authorisation shall provide him with a statement of his turnover for the previous calendar year; and, if specified in the demand, an audit certificate relating to the turnover.
(2) When he provides the statement of his turnover he shall pay an annual fee, rounded up to the next £10, of—
where
T is the annual turnover in the previous calendar year and n is the number of active marketing authorisations held at any time during the previous calendar year.
(3) In the case of an authorisation holder with a turnover relating to all marketing authorisations held of less than £220,000, the amount, rounded up to the next £10, is—
where
T and n mean the same as in the preceding sub-paragraph.
(4) In this paragraph—
“turnover” means the gross value at manufacturers' prices of all authorised veterinary medicinal products sold or supplied in the United Kingdom;
“manufacturers' prices” means the prices charged for authorised products by manufacturers to wholesalers, except to the extent that—
the products are supplied by manufacturers direct to retailers, in which case it means the prices charged for the products by the manufacturers to the retailers reduced by such sum as, in the opinion of the Secretary of State, represents the difference between the prices paid by the retailers and those which could be expected to be charged by the manufacturers to wholesalers according to the practice prevailing during the period in question with regard to such products;
a marketing authorisation holder sells or supplies products which he has neither manufactured nor obtained from the manufacturer, in which case it means the prices paid by him for those products.
Auditor’s certificate
22.—(1) If the Secretary of State required an audit certificate when he sent out the demand for the statement of turnover, and the holder of the marketing authorisation has not provided it within 30 days, an additional fee is payable for that year of £10,765 plus an additional £2,155 in respect of each marketing authorisation held.
(2) If the Secretary of State is not satisfied that the audit certificate provides sufficient assurance that the figures fairly present the financial records of the company, he shall require the marketing authorisation holder to produce within 30 days a further certificate and specify what further assurances he needs; and if these are not provided within those 30 days the additional fee specified in sub-paragraph (1) is payable.
(3) Nothing in this paragraph limits the powers of an inspector to examine financial records.
Late payment of annual fees
23.—(1) Where a person fails to pay the annual fee for a marketing authorisation within 30 days from and including the date of the demand, he must pay an additional fee, rounded up to the nearest £10, of—
(a)where payment is received after 30 but before 60 days have expired from and including the due date, 1% of the annual fee;
(b)where payment is received after 60 but before 90 days have expired from and including the due date, 2% of the annual fee; and
(c)where payment has not been received after the expiry of 90 days, 5% of the annual fee.
(2) Where a marketing authorisation holder has not provided the Secretary of State with a statement of his annual turnover so that the annual fee cannot be determined before the due date, he may make a payment of an amount on account of the annual fee, in which case the additional fee is calculated on the difference between the amount paid on account and the actual amount due.
The Committee was established by Article 30 of Regulation (EC) No. 762/2004 of the European Parliament and of the Council laying down Community procedures for the authorisation and supervision of medicinal products for human and veterinary use and establishing a European Medicines Agency, OJ No. L 136, 30.4.2004, p. 1.
Options/Help
Print Options
PrintThe Whole Instrument
PrintThe Whole Schedule
PrintThis Part only
You have chosen to open The Whole Instrument
The Whole Instrument you have selected contains over 200 provisions and might take some time to download. You may also experience some issues with your browser, such as an alert box that a script is taking a long time to run.
Would you like to continue?
You have chosen to open The Whole Instrument as a PDF
The Whole Instrument you have selected contains over 200 provisions and might take some time to download.
Would you like to continue?
You have chosen to open the Whole Instrument
The Whole Instrument you have selected contains over 200 provisions and might take some time to download. You may also experience some issues with your browser, such as an alert box that a script is taking a long time to run.
Would you like to continue?
You have chosen to open Schedules only
The Schedules you have selected contains over 200 provisions and might take some time to download. You may also experience some issues with your browser, such as an alert box that a script is taking a long time to run.
Would you like to continue?
Legislation is available in different versions:
Latest Available (revised):The latest available updated version of the legislation incorporating changes made by subsequent legislation and applied by our editorial team. Changes we have not yet applied to the text, can be found in the ‘Changes to Legislation’ area.
Original (As Enacted or Made): The original version of the legislation as it stood when it was enacted or made. No changes have been applied to the text.
Explanatory Memorandum
Explanatory Memorandum sets out a brief statement of the purpose of a Statutory Instrument and provides information about its policy objective and policy implications. They aim to make the Statutory Instrument accessible to readers who are not legally qualified and accompany any Statutory Instrument or Draft Statutory Instrument laid before Parliament from June 2004 onwards.
More Resources
Access essential accompanying documents and information for this legislation item from this tab. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as enacted version that was used for the print copy
- lists of changes made by and/or affecting this legislation item
- confers power and blanket amendment details
- all formats of all associated documents
- correction slips
- links to related legislation and further information resources
More Resources
Use this menu to access essential accompanying documents and information for this legislation item. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as made version that was used for the print copy
- correction slips
Click 'View More' or select 'More Resources' tab for additional information including:
- lists of changes made by and/or affecting this legislation item
- confers power and blanket amendment details
- all formats of all associated documents
- links to related legislation and further information resources