- Latest available (Revised)
- Original (As made)
The Poultry Meat, Farmed Game Bird Meat and Rabbit Meat (Hygiene and Inspection) Regulations 1995
You are here:
- UK Statutory Instruments
- 1995 No. 540
- Schedules only
More Resources
Status:
This is the original version (as it was originally made). This item of legislation is currently only available in its original format.
Regulations 4(2)(a), 8(1)(d)
SCHEDULE 1CONSTRUCTION, LAYOUT AND EQUIPMENT OF SLAUGHTERHOUSES (EXCEPT LOW THROUGHPUT SLAUGHTERHOUSES), CUTTING PREMISES (EXCEPT LOW THROUGHPUT CUTTING PREMISES) COLD STORES AND RE-WRAPPING CENTRES – GENERAL REQUIREMENTS
All slaughterhouses, cutting premises, cold stores and re-wrapping centres shall have—
1. in rooms where fresh meat is produced, worked on or stored and in areas and corridors through which such meat is transported—
(a) waterproof flooring which is easy to clean and disinfect, rotproof and laid in such a way as to facilitate the draining of water; the water shall be directed towards drains fitted with gratings and traps to prevent odours. However—
(i)in the case of rooms referred to in paragraph 1(e) of Schedule 2, paragraph 1(a) of Schedule 3 and paragraph 1(a) of Schedule 4, directing of water towards drains fitted with gratings and traps shall not be required and, in the case of premises referred to in paragraph 1(a) of Schedule 4, a device with which water may easily be removed shall be sufficient;
(ii)in the case of rooms referred to in paragraph 2(a) of Schedule 4 which store only wrapped or packaged meat and in areas and corridors through which fresh meat is transported, waterproof and rotproof flooring shall be sufficient;
(b) smooth, durable, impermeable walls, with a light-coloured, washable coating up to a height of at least two metres or the height of the room, if lower; in chilling or refrigeration rooms and in stores the walls shall be coated at least to storage height. Wall to floor junctions shall be rounded or similarly finished except in the rooms referred to in paragraph 2(a) of Schedule 4. However, in the rooms referred to in paragraph 2 of Schedule 4 and provided they were built before 1st January 1994, the interior walls may be made of wood;
(c) door and window frames in hard-wearing, non-corrodible material and, if made of wood, with a smooth and impermeable covering on all surfaces;
(d) insulation materials which are rotproof and odourless;
(e) adequate ventilation and extraction of steam;
(f) adequate natural or artificial lighting which does not distort colours;
(g) a clean and easily cleaned ceiling; if there is no ceiling, a roof covering with an interior surface which fulfils these conditions.
2. save for cold stores handling only wrapped and packaged meat—
(a) as near as possible to, or readily accessible to, the work stations, a sufficient number of facilities for cleaning hands and for cleaning tools with hot water, with taps which are not hand-operable or arm-operable. These facilities shall have hot and cold running water or water premixed to a suitable temperature for washing hands, cleaning products and hygienic means of drying hands; and
(b) facilities for disinfecting tools, with water at not less than 82°C.
3. adequate arrangements for protection against pests such as insects and rodents.
4.—(a) instruments and working equipment such as automatic equipment for working on meat, cutting tables, tables with detachable cutting surfaces, containers, conveyor belts and saws, made of corrosion-resistant material not liable to taint meat and easy to clean and disinfect. Surfaces coming into, or likely to come into contact with meat, including welds and joins, shall be maintained smooth. Without prejudice to paragraph 1(b) above, no wood shall be used except in rooms where the only fresh meat stored is hygienically packaged fresh meat;
(b) corrosion-resistant fittings and equipment meeting hygiene requirements for—
(i)meat handling; and
(ii)storing meat, in such a way that neither the meat nor the containers come into direct contact with the floor or walls;
(c) facilities, including suitably laid out and equipped reception and marshalling areas, for the hygienic handling and protection of meat during loading and unloading;
(d) designated watertight non-corrodible containers, with lids and fasteners to prevent unauthorised persons from removing things from them, for keeping meat not intended for human consumption, or a lockable room for such meat if the quantities are large enough to necessitate this or if the meat is not removed or destroyed at the end of each working day; where such meat is removed through conduits, these shall be so constructed and installed as to avoid any risk of contamination of the fresh meat; and
(e) a room or rooms for the hygienic storage of materials for wrapping and packaging where such activities are carried out in the establishment.
5. refrigeration equipment to keep the internal temperature of the meat at the levels required by Schedule 12, such equipment to have a drainage system which minimises the risk of contamination of fresh meat.
6. an adequate pressurised supply of potable water within the meaning of Directive (1). Non-potable water pipes shall be clearly distinguished from those used for potable water.
7. an adequate supply of hot potable water within the meaning of Directive 80/778/EEC.
8. a liquid and solid waste disposal system which meets hygiene requirements.
9. adequate lockable facilities for the exclusive use of the official veterinary surgeon and the inspectors.
10. facilities enabling the required veterinary inspections to be carried out efficiently at any time.
11. an adequate number of changing rooms with smooth, waterproof, washable walls and floors, wash basins, showers and flush lavatories so equipped as to protect the clean parts of the building from contamination. The lavatories shall not open directly on to the work rooms. Showers shall not be necessary in cold stores receiving and storing hygienically wrapped or packaged fresh meat only. Wash basins shall have hot and cold running water or water premixed to a suitable temperature, materials for cleaning hands and hygienic means of drying hands. Wash basin taps shall not be hand-operable or arm-operable. There shall be a sufficient number of such wash basins near the lavatories.
12. a place and adequate facilities for cleaning and disinfecting means of transport for meat except in the case of cold stores receiving and storing hygienically packaged fresh meat only. Slaughterhouses shall have a separate place and separate facilities for cleaning and disinfecting means of transport and crates used for birds or rabbits intended for slaughter. However, these places and facilities shall not be compulsory if alternative facilities which are approved by the official veterinary surgeon, are available for the means of transport or crates to be cleaned and disinfected.
13. a suitable room or secure place for the storage of detergents, disinfectants and similar substances.
Regulations 4(2)(a), 8(1)(d)
SCHEDULE 2CONSTRUCTION, LAYOUT AND EQUIPMENT OF SLAUGHTERHOUSES (EXCEPT LOW THROUGHPUT SLAUGHTERHOUSES) – ADDITIONAL REQUIREMENTS
1. In addition to the general requirements contained in Schedule 1, slaughterhouses shall have—
(a) a room or covered space which is sufficiently large and easy to clean and disinfect for the pre-slaughter inspection provided for in paragraph 9 of Schedule 7;
(b) a slaughter room large enough for stunning and bleeding on the one hand, and plucking and any scalding of any birds and skinning of any rabbits on the other, to be carried out in separate places. Any communication between the slaughter room and the room or space referred to in sub-paragraph (a) above other than the narrow opening through which only slaughter birds or rabbits may pass shall have an automatically closing door;
(c) an evisceration and preparation room which is large enough for evisceration to be carried out in a place sufficiently far from the other work stations, or separated from them by a partition, so as to prevent contamination. Any communication between the evisceration and preparation room and the slaughter room other than the narrow opening through which only slaughtered birds or rabbits may pass shall have an automatically closing door;
(d) where necessary, a dispatching room;
(e) one or more sufficiently large chilling or refrigerating rooms, with a lockable facility for fresh meat which has been detained;
(f) in the case of premises handling birds a room or space for collecting feathers except where these are treated as waste;
(g) for staff handling live birds or live rabbits, either—
(i)separate wash basins and lavatories; or
(ii)arrangements to ensure that such staff wash their hands and change their protective clothing before using the changing rooms required by paragraph 11 of Schedule 1.
Regulations 4(2)(a), 8(1)(d)
SCHEDULE 3CONSTRUCTION, LAYOUT AND EQUIPMENT OF CUTTING PREMISES (EXCEPT LOW THROUGHPUT CUTTING PREMISES) – ADDITIONAL REQUIREMENTS
1. In addition to the general requirements contained in Schedule 1, cutting premises shall have—
(a) chilling or refrigerating rooms large enough for meat preservation;
(b) a room for cutting up, boning and wrapping and, in so far as this operation is carried out in those cutting premises, a room for the evisceration of birds referred to in paragraph 4 of Part I of Schedule 9;
(c) a room for packaging operations, where such operations are carried out in those cutting premises, unless the conditions provided for in paragraph 5 of Schedule 13 are fulfilled.
Regulations 4(2)(a), 8(1)(d)
SCHEDULE 4CONSTRUCTION, LAYOUT AND EQUIPMENT OF COLD STORES – ADDITIONAL REQUIREMENTS
1. In addition to the general requirements contained in Schedule 1, stores in which fresh meat is stored in accordance with paragraph 1 of Schedule 12 shall have—
(a) sufficiently large chilling and refrigeration rooms, which are easy to clean and in which fresh meat can be stored at the temperature provided for in paragraph 1 of Schedule 12;
(b) a recording thermometer or recording telethermometer in or for each storage area.
2. In addition to the general requirements contained in Schedule 1, stores in which fresh meat is stored in accordance with paragraph 2 of Schedule 12 shall have—
(a) sufficiently large chilling and refrigerating rooms, which are easy to clean and in which fresh meat can be stored at the temperature provided for in paragraph 2 of Schedule 12;
(b) a recording thermometer or recording telethermometer in or for each storage area.
Regulations 4(2)(a), 8(1)(d)
SCHEDULE 5CONSTRUCTION, LAYOUT AND EQUIPMENT OF LOW THROUGHPUT SLAUGHTERHOUSES AND LOW THROUGHPUT CUTTING PREMISES
PART IGENERAL REQUIREMENTS
All low throughput slaughterhouses and low throughput cutting premises shall have—
1. in rooms where fresh meat is produced and worked on—
(a) waterproof flooring which is easy to clean and disinfect, rotproof and laid in such a way as to facilitate the draining of water; the water shall be directed towards drains fitted with gratings and traps to prevent odours. However, in the case of rooms referred to in paragraph 10 below, a device with which water may easily be removed shall be sufficient;
(b) smooth, durable, impermeable walls, with a light-coloured, washable coating up to a height of at least two metres, or the height of the room, if lower. However, in the rooms referred to in paragraph 1 of Schedule 4 and provided they were built before 1st January 1994, the interior walls may be made of wood;
(c) doors in easily cleanable, rotproof and odourless material;
(d) insulation materials which are rotproof and odourless;
(e) adequate ventilation and, if necessary, extraction of steam; and
(f) adequate natural or artificial lighting which does not distort colours.
2.—(a) as near as possible to, or readily accessible to, the work stations, a sufficient number of facilities for cleaning hands and for cleaning tools with hot water. These facilities shall have hot and cold running water or water premixed to a suitable temperature for washing hands, cleaning products and hygienic means of drying hands;
(b) facilities in the same room or in an adjacent room for disinfecting tools, with hot water supplied at not less than 82°C.
3. adequate arrangements for protection against pests such as insects and rodents.
4.—(a) instruments and working equipment such as cutting tables, tables with detachable cutting surfaces, containers, conveyor belts and saws, made of corrosion-resistant material, not liable to taint meat and easy to clean and disinfect. Without prejudice to paragraph 1(b) above, no wood shall be used;
(b) corrosion-resistant fittings and equipment meeting hygiene requirements for—
(i)meat handling; and
(ii)storing meat, in such a way that neither the meat nor the containers come into direct contact with the floors or walls; and
(c) designated watertight non-corrodible containers, with lids and fasteners to prevent unauthorised persons from removing things from them, for keeping meat not intended for human consumption; such meat shall be removed or destroyed at the end of each working day.
5. refrigeration equipment to keep the internal temperature of the meat at the levels required by Schedule 12; such equipment to have a drainage system which minimises the risk of contamination of the meat. However neither requirement shall apply where meat is removed immediately from that low throughput slaughterhouse for delivery to cutting plants or butcher shops in its immediate vicinity and such transportation does not take more than one hour.
6. an adequate pressurised supply of potable water within the meaning of Directive 80/778/EEC. Non-potable water pipes shall be clearly distinguished from those used for potable water.
7. an adequate supply of hot potable water within the meaning of Directive 80/778/EEC.
8. an adequate system for the hygienic disposal of waste water.
9. at least one wash basin and flush lavatory. The latter shall not open directly onto the work rooms. The wash basin shall have hot and cold running water or water premixed to a suitable temperature, materials for cleaning hands and hygienic means of drying hands. The wash basin shall be near the lavatory.
10.—(a) storage facilities which, subject to sub-paragraph (b) below, satisfy the requirements of paragraphs 1(a), (b) and (c) above, where meat is stored in those premises;
(b) in place of the drain referred to in paragraph 1(a) such facilities may have a device, other than a drain, with which water may easily be removed.
PART IILOW THROUGHPUT SLAUGHTERHOUSES – ADDITIONAL REQUIREMENTS
11.—(a) In addition to the general requirements contained in Part I of this Schedule, low throughput slaughterhouses shall have—
(i)a slaughter room large enough for stunning and bleeding on the one hand, and plucking and any scalding of birds or skinning of rabbits on the other, to be carried out in separate places;
(ii)an evisceration and preparation room which is large enough for evisceration to be carried out in a place sufficiently far from other work stations, or separated from them by a partition, so as to prevent contamination;
(b) notwithstanding sub-paragraph (a) of this paragraph, the room used for the slaughter of birds or rabbits may also be used for the evisceration and preparation of such birds or rabbits after such room has been cleaned thoroughly and disinfected after its use for each such operation, so as to minimise the risk of any contamination of meat.
PART IIILOW THROUGHPUT CUTTING PREMISES CARRYING OUT DELAYED EVISCERATION – ADDITIONAL REQUIREMENTS
12. In addition to the general requirements contained in Part I of this Schedule, low throughput cutting premises carrying out delayed evisceration shall meet the requirements contained in Schedule 3 to these Regulations save that, notwithstanding the requirement set out in paragraph 1 of Schedule 3, the room used for cutting up, boning and wrapping of birds may also be used for the evisceration and preparation of birds provided that the room has been cleaned thoroughly and disinfected following its use for each such operation, so as to minimise the risk of contamination of meat.
Regulations 4(2)(a), 8(1)(d)
SCHEDULE 6HYGIENE REQUIREMENTS IN RELATION TO STAFF, PREMISES, EQUIPMENT AND IMPLEMENTS
Requirements applicable to all premises
1. The occupier of any premises shall keep them, or cause them to be kept, in such a state of cleanliness and otherwise so conduct them as to prevent any risk of contamination of any fresh meat therein.
2.—(a) Staff handling exposed or wrapped fresh meat or working in rooms and areas in which such meat is handled, or transported shall wear clean and easily cleanable headgear, footwear and light-coloured working clothes or other protective clothing. Staff engaged in working on or handling fresh meat shall wear clean working clothes at the commencement of each working day and shall renew such clothing during the day as necessary and wash their hands several times during the working day and each time work is resumed. Persons who have been in contact with sick birds or sick rabbits or infected meat shall immediately afterwards carefully wash their hands and arms with hot water. No person shall smoke in work rooms and store rooms and in other areas and corridors through which fresh meat is transported.
(b) No animal shall be allowed to enter the establishments except, in the case of slaughterhouses, animals for slaughter. Rodents, insects and other vermin shall be systematically destroyed.
(c) Equipment and instruments used for handling live birds or rabbits and working on fresh meat shall be kept clean and in a good state of repair. They shall be carefully cleaned and disinfected several times during the working day, at the end of the day’s work and before being re-used when they have been soiled.
(d) Crates for delivering birds or rabbits shall be made of non-corrodible material, easy to clean and disinfect. They shall be cleaned and disinfected each time they are emptied.
3. Rooms, instruments and working equipment shall not be used for purposes other than work on fresh meat or fresh meat within the meaning of regulation 2(1) of the Fresh Meat (Hygiene and Inspection) Regulations 1995(2) or wild game meat within the meaning of Council Directive 92/45/EEC or meat preparations or products unless they are cleaned and disinfected before re-use. However, this restriction shall not apply to transport equipment used in relation to meat which is packaged.
4. Meat and containers thereof shall not come into direct contact with the floor.
5. Potable water shall be used for all purposes; however, non-potable water may be used for steam production, fire fighting, cooling refrigeration equipment and removing waste feathers in the slaughterhouse provided that the pipes installed for this purpose preclude the use of such water for other purposes and present no danger of contamination of fresh meat.
6. Sawdust or any other similar substance shall not be spread on the floor of the workrooms or fresh meat storage rooms.
7. Detergents, disinfectants and similar substances shall be used in such a way that instruments, working equipment and fresh meat are not adversely affected. Their use shall be followed by thorough rinsing of such instruments and working equipment with potable water, as necessary, except where the disinfectant or similar substance is specially designed not to be rinsed after application.
9. On being recruited, any person working on or handling unpackaged fresh meat shall be required to prove, by a medical certificate, that there is no objection on public health grounds to his engagement in such activities and every such medical certificate shall be produced on request to an official veterinary surgeon or to a veterinary officer.
Regulations 4(2)(a), 8(1)(a), 12(1)(a), 14(1)(b), 21
SCHEDULE 7PRE-SLAUGHTER HEALTH INSPECTION
1. Subject to paragraph 2 below, the official veterinary surgeon shall authorise the slaughter of a specified group from a holding where—
(a) the birds or rabbits intended for slaughter are accompanied by a health attestation in the form specified in Schedule 17; or
(b) not less than 72 hours before the arrival of such birds or rabbits at the slaughterhouse, the official veterinary surgeon was in possession of—
(i)a declaration from a veterinary surgeon that the holding is under his supervision, and
(ii)the information specified in Part I of Schedule 22 relating to the birds or rabbits in question, ascertained from the records kept by the producer of those birds or rabbits, as the case may be, and set out in a report signed or otherwise authenticated by that producer;
(iii)such additional information, as the official veterinary surgeon may request, relating to such birds or rabbits which may include the details specified in Part II of Schedule 22.
2. The official veterinary surgeon shall assess the information referred to in paragraph 1(b) above, supplied by the producer, in determining the steps to be taken with regard to the birds or rabbits coming from the holding of that producer, in particular the type of any pre-slaughter health inspection to be carried out on such birds or rabbits.
3. Where the conditions specified in paragraph 1 above are not satisfied, the official veterinary surgeon of the slaughterhouse may—
(a) postpone the slaughter, or
(b) where he feels it necessary to do so for reasons of animal welfare—
(i)authorise the slaughter after an inspection of the birds or rabbits in accordance with paragraph 7(b) below, and
(ii)arrange for an inspection by an official veterinary surgeon or the veterinary surgeon referred to in paragraph 1(b)(i) above, of the holding of origin of the birds or rabbits in question to ascertain the information referred to in paragraph 1(b) above.
4. In the case of producers who annually fatten or rear not more than 20,000 domestic fowl, 15,000 ducks, 10,000 turkeys, 10,000 geese, 20,000 rabbits, or an equivalent quantity of other species of bird, the pre-slaughter inspection may be carried out at the slaughterhouse. In such case the producer shall provide a declaration to the effect that his annual production does not exceed the said figures.
5. The producer shall retain the records referred to in paragraph 7(a) below for a period of two years from the date to which such records relate and upon demand by the Minister submit them to the Minister.
6. The pre-slaughter health inspection shall be carried out in adequate lighting.
7. The pre-slaughter health inspection of birds and rabbits on their farm of origin shall comprise—
(a) checking of the producers' records which, depending on the type of birds or rabbits, shall include the following, save that the details specified at items (iv), (viii) and (xii) shall not be required unless specified in writing by the Minister in relation to any particular birds or rabbits:
(i)day of arrival of the birds or rabbits,
(ii)source of the birds or rabbits,
(iii)number of birds or rabbits,
(iv)actual performance of the particular breeds (e.g. weight gain),
(v)mortality,
(vi)suppliers of feedingstuffs,
(vii)type, period of use and withdrawal periods of feed additives,
(viii)consumption of feedingstuffs and water,
(ix)examination and diagnosis of the attending veterinary surgeon engaged by that producer, together with any laboratory results,
(x)type of any medicinal product, with dates of administration and withdrawal, given to the birds or rabbits,
(xi)date and type of any vaccines given,
(xii)weight gain during the fattening period,
(xiii)results of any previous official pre-slaughter health inspections of birds from the same specified group,
(xiv)number of birds or rabbits sent for slaughter,
(xv)expected date of slaughter;
(b) any additional examinations needed to establish whether the birds or rabbits—
(i)are suffering from a disease which can be transmitted to humans or to animals or are behaving, on an individual or collective basis, in such a way as to indicate that such a disease may occur,
(ii)show disturbance of general behaviour or signs of sickness which may make the meat unfit for human consumption;
(c) regular sampling of water and feed with a view to checking compliance with withdrawal periods;
(d) the results of tests for zoonotic agents carried out in accordance with Directive 92/117/EEC(3).
8. At the slaughterhouse, the official veterinary surgeon shall take all necessary steps to ensure that the birds or rabbits are identified, that the requirements of Chapter II of Directive (4) have been complied with and that the birds or rabbits are checked for injury during transport.
9. In the case of there being any doubt concerning the identity of a consignment of birds or rabbits or where the birds or rabbits are required to undergo a pre-slaughter health inspection at the slaughterhouse in accordance with paragraph 4 above, the official veterinary surgeon shall examine each crate of the birds or rabbits if the birds or rabbits, as the case may be, show the signs referred to in paragraph 7(b) above.
10. Where any birds or rabbits have not been slaughtered within three days of their examination and the issue of the health attestation provided for in paragraph 1(a) above—
(a) where the birds or rabbits have not left the holding of origin, a new health attestation (in the form specified in Schedule 17) shall be required, or
(b) the official veterinary surgeon of the slaughterhouse may, after considering the reasons for the delay in slaughtering and on being satisfied, following such further examination he may consider to be necessary of the birds or rabbits, as the case may be, that there is no health risk, authorise the slaughter of those birds or rabbits.
11. Without prejudice to the requirements of Directive 91/494/EEC, no bird shall be slaughtered for the purpose of human consumption if the clinical signs of the following diseases have been established:
(a) ornithosis,
(b) salmonellosis.
12. The official veterinary surgeon may, at the request of the producer of the birds referred to in paragraph 11 above, authorise the slaughter of such birds at the end of the current slaughtering period at the slaughterhouse provided that precautions are taken to keep to a minimum the risk of spreading pathogenic micro-organisms and to clean and disinfect the facilities after the slaughter, with the meat of the slaughtered birds being handled as if it were meat declared unfit for human consumption.
13. The official veterinary surgeon shall—
(a) prohibit slaughter where he has evidence that the meat from the birds concerned would be unfit for human consumption;
(b) postpone slaughter where the withdrawal period for residues has not been respected;
(c) ensure, with regard to clinically healthy poultry from a specified group the slaughter of which is obligatory under a programme for the control of infectious disease, that those birds are slaughtered at the end of the current slaughtering period or under conditions such that any contamination of other birds is avoided.
14. The official veterinary surgeon shall immediately notify the Minister of any prohibition of slaughter, giving reasons, and provisionally place the birds affected by such slaughter prohibition in safekeeping.
15. Sick or suspect birds or rabbits shall not be slaughtered in the premises except in accordance with an authorisation granted by the official veterinary surgeon. Where such an authorisation is granted, slaughter shall be performed under the supervision of the official veterinary surgeon and steps taken to prevent contamination; the premises shall be specially cleaned and disinfected under such supervision before being used again.
16. No rabbit shall be slaughtered for human consumption if it has been established that—
(a) the rabbit is suffering from a disease which can be transmitted to humans or animals, or shows clinical signs of such a disease, or the general condition of the rabbit is such as to indicate that such a disease may occur; or
(b) the rabbit shows clinical signs of a disease or disorder affecting its general condition which may make the meat unfit for human consumption.
17. Any rabbit referred to in paragraph 16 above shall be slaughtered either separately or after the slaughter of all other rabbits, with the meat of that slaughtered rabbit being hygienically disposed of.
Regulations 4(2)(a), 8(1)(d), 14(1)(c)
SCHEDULE 8HYGIENE REQUIREMENTS FOR SLAUGHTER AND THE HANDLING OF FRESH MEAT
1.—(a) Only live birds or rabbits may be brought into a slaughterhouse. The birds or rabbits shall, except in the case of slaughter according to a religious rite, be stunned and immediately slaughtered.
(b) Notwithstanding sub-paragraph (a) above, dead small wild game may be brought into a slaughterhouse as permitted by Article 3(1)(a) and (b)(ii) of Council Directive 92/45/EEC.
2. Bleeding shall be completed and carried out in such a way that the blood cannot cause contamination outside the place of slaughter.
3. Slaughtered birds shall be plucked completely before skinning or cutting and slaughtered rabbits shall be skinned immediately.
4. Evisceration shall be carried out immediately in the case of total or partial evisceration or within the period laid down in paragraph 4 of Part I of Schedule 9 in the case of deferred evisceration. Slaughtered birds or rabbits shall be opened in such a way that the cavities and all the relevant viscera can be inspected. For this purpose the viscera to be inspected may either be detached or left attached to the carcase by their natural connections. If detached, they shall be identifiable as belonging to a given carcase.
5. After inspection, the viscera which has been removed shall be separated immediately from the carcase, and the parts unfit for human consumption removed at once. Viscera or parts of viscera remaining in the carcase shall, with the exception of the kidneys, be removed entirely if practicable, under satisfactory hygienic conditions.
6. Meat shall not be cleaned by wiping with a cloth and the carcase shall not be filled with anything other than edible offal or neck offal from birds or rabbits slaughtered in the slaughterhouse which shall be wrapped in accordance with paragraph 2(c) of Schedule 13.
7. The carcase shall not be cut into portions and the meat shall not be removed or subjected to any process prior to the post-mortem health inspection except with the consent of the official veterinary surgeon who may prescribe any other handling required for the purposes of the post-mortem health inspection.
8. Detained meat, on the one hand, and meat declared unfit for human consumption in accordance with paragraph 1 of Part II of Schedule 9 or not allowed for human consumption in accordance with paragraph 2 of that Part on the other, and feathers and waste shall be removed as soon as possible to the rooms, facilities or containers referred to in paragraph 4(d) of Schedule 1 and paragraph 1(e) and (f) of Schedule 2 and shall be handled in such a way so as to ensure that contamination is kept to a minimum.
9. After inspection and evisceration fresh meat shall immediately be cleaned and chilled hygienically to ensure compliance with the temperature laid down in Schedule 12 as soon as possible.
10.—(a) Birds subjected to delayed evisceration shall be chilled hygienically immediately after plucking. They shall not be chilled by an immersion chilling process.
(b) Carcases of birds to be subjected to an immersion chilling process in accordance with the process described in paragraph 11 below shall, immediately after evisceration, be thoroughly washed by spraying and immersed without delay. The spraying shall be carried out by means of equipment which washes both the internal and external surfaces of the carcases efficiently and in accordance with the requirements of paragraph (c) below.
(c) For carcases weighing—
(i)not more than 2.5 kg, at least 1.5 litres of water shall be used per carcase,
(ii)between 2.5 kg and 5 kg, at least 2.5 litres of water shall be used per carcase,
(iii)5 kg or more, at least 3.5 litres of water shall be used per carcase.
11. The immersion chilling process shall meet the following requirements—
(a) the carcases shall pass through one or more tanks of water or of ice and water, the contents of which are continuously renewed. Only a system whereby the carcases are constantly propelled by mechanical means through a counterflow of water shall be used;
(b) the temperature of the water in the tank or tanks measured at the points of entry and exit of the carcases shall not be more than +16°C and +4°C respectively;
(c) it shall be carried out in such a way that the temperature specified in Schedule 12 is reached in the shortest possible time;
(d) the minimum flow of water throughout the whole chilling process referred to in sub-paragraph (a) of this paragraph shall be—
(i)2.5 litres per carcase weighing 2.5 kg or less,
(ii)4 litres per carcase weighing between 2.5 kg and 5 kg,
(iii)6 litres per carcase weighing 5 kg or more;
(e) where there are several tanks, the inflow of fresh water and the outflow of used water in each tank shall be regulated in such a way as to progressively decrease in the direction of movement of the carcases, the fresh water being divided between the tanks in such a way that the flow of water through the last tank is not less than—
(i)1 litre per carcase weighing 2.5 kg or less,
(ii)1.5 litres per carcase weighing between 2.5 kg and 5 kg,
(iii)2 litres per carcase weighing 5 kg or more.
The water used for first filling the tanks shall not be included in the calculation of these quantities;
(f) the carcases shall not remain in the first part of the apparatus or the first tank for more than half an hour or in the rest of the apparatus or the other tank or tanks for longer than is strictly necessary;
(g) all necessary precautions shall be taken to ensure that, in the event of interruptions of the process, the transit time laid down in the sub-paragraph (f) of this paragraph is complied with; whenever there has been a stoppage of the equipment, the official veterinary surgeon shall satisfy himself prior to re-setting it in motion that the carcases still meet the requirements of these Regulations and are fit for human consumption and, if such is not the case, ensure that they are transported as soon as possible to the facilities provided for in paragraph 4(d) of Schedule 1;
(h) each piece of equipment shall be entirely emptied, cleaned and disinfected whenever this is necessary at the end of the period of work and at least once a day;
(i)calibrated control equipment shall be used that permits adequate and continued supervision of the measuring and recording of—
(i)the water consumption during spray-washing before immersion,
(ii)the temperature of the water in the tank or tanks at the points of entry and exit of the carcases,
(iii)the water consumption during immersion,
(iv)the number of carcases in each of the weight-ranges listed in sub-paragraphs (d) and (e) of this paragraph and paragraph 10 above;
(j) the results of the various checks carried out by the producer shall be kept and submitted on request to the official veterinary surgeon;
(k) the correct functioning of the chilling plant and its effect on the hygiene level shall be evaluated by scientific microbiological methods, the contamination of the carcases with total bacteria and enterobacteria being compared before and after immersion. Such comparison shall be carried out when the plant is first brought into use and after that periodically and in any case each time any alterations are made to the plant. The functioning of the various parts shall be regulated so as to ensure a satisfactory standard of hygiene;
(l) notwithstanding the preceding sub-paragraphs of this paragraph, in the case of poultry slaughtered by the Jewish method for the food of Jews, salting and subsequent washing off of the salt, carried out under the supervision of the Local Board of Shechita or, in the absence of any such Board, of a Committee appointed for the purpose by the local Jewish congregation established in accordance with Jewish law, may take place immediately after the carcases emerge from the first part of the equipment or, where the equipment comprises more than one tank, the first tank. In such cases the period in the first part of the equipment or first tank may be extended to 40 minutes. Any immersion of the carcases in water for the purposes of soaking, salting and subsequent washing off of the salt shall comply with the requirements of sub-paragraphs (a), (d), (e) and (h) of this paragraph.
12. Adequate arrangements shall be made so as to ensure that until the inspection has been completed, it shall not be possible for carcases and offal not yet inspected to come into contact with carcases and offal already inspected and that there is no removal, cutting or further treatment of the carcase.
13. Adequate arrangements shall be made so as to ensure that it shall not be possible for meat detained or declared unfit for human consumption or inedible by-products to come into contact with meat declared fit for human consumption, and the former shall be placed as soon as possible in special rooms or containers located and laid out in such a way as to avoid any contamination of other fresh meat.
14. The drawing and trussing, handling, further treatment and transport of meat, including offal, shall be performed in accordance with all hygiene requirements. Where such meat is packaged, the requirement in paragraph 1(d) of Schedule 2 and the conditions laid down in Schedule 13 shall be complied with. Packaged meat shall be stored in a separate room from exposed fresh meat.
Regulations 4(2)(a), 8(1)(b), 11(1), 12(1)(b), 14(1)(d)
SCHEDULE 9POST-MORTEM HEALTH INSPECTION
PART IGENERAL REQUIREMENTS
1. The birds and rabbits shall be inspected immediately after slaughter under suitable lighting and as part of such inspection—
(a) in the case of birds, the following parts:
(i)the surface of the bird’s body, excluding head and feet save where these are intended for human consumption;
(ii)the viscera; and
(iii)the body cavities,
shall be subjected to visual inspection and, where necessary, palpation and incision, except that where, in the case of quail and pigeon, the evisceration technique does not permit complete inspection of the viscera of each bird, that inspection may be carried out on a sample of at least 5% of each batch of such birds, the opinion expressed as to the fitness for human consumption of the sample inspected applying to the whole batch.
(b) in the case of rabbits, the following shall be carried out:
(i)visual inspection of the slaughtered animal;
(ii)where necessary, palpation and incision of the lungs, liver, spleen, kidneys and parts of the carcase which have undergone any change;
(iii)investigation of anomalies of consistency, colour, smell and, where appropriate taste; and
(iv)where necessary, laboratory tests.
(c) attention shall also be paid to:
(i)anomalies of consistency, colour and smell in the carcases;
(ii)major anomalies resulting from slaughtering operations; and
(iii)proper functioning of the slaughter equipment.
2. In the case of birds, the official veterinary surgeon shall in any event:
(a) subject to detailed inspection a random sample of the birds rejected in the post-mortem health inspection, the meat of which was declared unfit for human consumption in accordance with paragraph 1 of Part II of this Schedule;
(b) examine a random sample of birds taken from the specified group which has undergone the post-mortem inspection, for an inspection of the viscera and the body cavities;
(c) carry out a special post-mortem inspection of the poultrymeat if there are other indications that the meat from that poultry could be unfit for human consumption.
3. In the case of partly eviscerated poultry (“effileé”) whose intestines have been removed immediately, the viscera and the body cavities of at least 5% of the slaughtered poultry from each specified group shall be inspected after evisceration. If during such inspection anomalies are discovered in a number of birds, then all the birds in the specified group shall be inspected in accordance with paragraph 1 above.
4. In the case of birds subjected to delayed evisceration:
(a) the post-mortem health inspection in accordance with paragraph 1 above shall take place at the latest 15 days after slaughter, during which period they must be kept at a temperature not exceeding +4°C;
(b) at the end of that period at the latest, they shall be eviscerated in the slaughterhouse where the slaughtering was performed, in an approved cutting plant fulfilling the additional requirements in paragraph 1(b) of Schedule 3 or in a low throughput cutting premises fulfilling the additional requirements of Part III of Schedule 5 and in the last two cases, be accompanied by the health certificate shown in Schedule 20;
(c) the poultry meat or farmed game meat shall not bear the health mark referred to in Schedule 11 before the evisceration referred to in sub-paragraph (b) of this paragraph has been performed.
5. The taking of samples to examine for residues shall be carried out by spot checks and in any case in the event of any justified suspicion. In the case of examination for residues by sampling, examination shall be carried out for the residues referred to in Group A III and Group B I(a) and (c) and II(a) of Annex 1 to Directive 86/469/EEC(5), as amended by Decision 89/187/EEC(6).
6. The obligation to examine for residues of substances with pharmacological action referred to in the second sub-paragraph of Article 4(1) of Directive 71/118/EEC shall not apply to poultry from holdings under official veterinary control where examination for those residues is carried out on the holdings of origin.
7. Where a disease is suspected on the basis of the pre-slaughter health inspection or post-mortem health inspection, the official veterinary surgeon may ask for the requisite laboratory tests to be carried out if he considers them necessary to substantiate his diagnosis or to detect substances with pharmacological action likely to be present, given the pathological condition observed. In case of any doubt, the official veterinary surgeon may perform any further cuts and inspections of the relevant parts of the birds or rabbits necessary in order to reach a definitive diagnosis.
PART IIINDICATIONS OF UNFITNESS FOR HUMAN CONSUMPTION
1.—(a) Birds shall be declared totally unfit for human consumption where the post-mortem health inspection reveals any of the following diseases or conditions:
generalized infectious disease and chronic localization in organs of pathogenic micro-organisms transmissible to humans;
systematic mycosis and local lesions in organs suspected of having been caused by pathogenic agents transmissible to humans or their toxins;
extensive subcutaneous or muscular parasitism and systematic parasitism;
poisoning;
cachexia;
abnormal smell, colour or taste;
malignant or multiple tumours;
general soiling or contamination;
major lesions and ecchymosis;
extensive mechanical lesions, including those due to extensive scalding;
insufficient bleeding;
residues of substances exceeding the authorised standards or residues of prohibited substances; or
ascites.
(b) Parts of a slaughtered bird which show localized lesions or contaminations not affecting the health of the rest of the meat shall be declared unfit for human consumption.
2. In the case of birds, the head separated from the carcase with the exception of the tongue, comb, wattles and caruncles and the following viscera shall be excluded from use for human consumption: trachea, lungs and crop separated from the carcase in accordance with paragraph 5 of Schedule 8, oesophagus, intestine and gall bladder.
3.—(a) Rabbits shall be declared totally unfit for human consumption where the post-mortem health inspection reveals any of the following diseases or conditions:
diseases transmissible to man or animals;
malignant or multiple tumours; multiple abscesses;
extensive parasitic infestation in the subcutaneous or muscle tissues;
presence of residues of forbidden substances or residues in excess of permitted Community levels, including substances with a pharmacological effect;
poisoning;
extensive injuries or extensive blood or serum imbibition;
anomalies as regards colour, smell or taste; or
anomalies as regards consistency, particularly oedema or severe emaciation.
(b) Parts of slaughtered rabbits which show localized lesions or contaminations not affecting the health of the rest of the meat shall be declared unfit for human consumption.
Regulations 4(2)(a), 8(1)(d)
SCHEDULE 10PROVISIONS CONCERNING MEAT INTENDED FOR CUTTING
1. The carcase shall be cut up into parts and boned only in licensed cutting premises.
2. The occupier shall facilitate operations for the supervision of the plant, in particular any handling which is considered necessary, and shall place the necessary facilities at the disposal of the persons carrying out such supervision under these Regulations. In particular, he shall on request inform the official veterinary surgeon responsible for supervision, of the source of the meat brought into his cutting plant and the origin of the birds or rabbits slaughtered.
3. Meat which does not fulfil the requirements of these Regulations shall not be placed in licensed cutting premises except in special storage areas; it shall be cut up in other places or at other times than meat which fulfils those requirements. The official veterinary surgeon shall at all times have access to all storage rooms and work rooms in order to satisfy himself that the preceding provisions of this Schedule are rigorously observed.
4. Fresh meat intended for cutting shall, as soon as it is brought in, be placed in the cutting room or, until cut up, in the room provided for in paragraph 1(a) of Schedule 3. However, notwithstanding paragraph 9 of Schedule 8, meat may be transported directly from the slaughter room to the cutting room. In such cases the slaughter room and the cutting room shall be sufficiently near to each other and located in the same group of buildings, since the meat to be cut shall be transferred in one operation from one room to the other by means of an extension of the mechanical handling system from the slaughter room, and cutting shall be carried out immediately. As soon as the prescribed cutting and packaging are completed, the meat shall be transported to the chilling room provided for in paragraph 1(a) of Schedule 3.
5. Meat shall be brought into the rooms referred to in paragraph 1(b) of Schedule 3 as required. As soon as cutting and, where appropriate, packaging are completed, the meat shall be transported to the chilling room provided for in paragraph 1(a) of Schedule 3.
6. Except in the case of meat cut while warm in accordance with paragraph 4 above, cutting may take place only if the meat has reached a temperature not exceeding +4°C.
7. Fresh meat shall not be cleaned by wiping with a cloth.
8. Cutting shall be carried out in such a way so as to avoid any soiling of the meat. Splinters of bone and clots of blood shall be removed. Meat obtained from cutting and not intended for human consumption shall be collected in the containers or rooms referred to in paragraph 4(d) of Schedule 1 as it is cut.
Regulations 8(1)(c), 12(2), 14(1)(e), (2)(a), (3)(c), 15(1)(c)
SCHEDULE 11HEALTH MARKING
1. Subject to paragraph 4 below, the health mark shall include:
(a) for meat wrapped in individual units or for small packages—
(i)on the upper part, the initials “UK”;
(ii)in the centre, the veterinary approval number of the establishment or, where appropriate, the cutting premises or re-wrapping centre; and
(iii)on the lower part, the initials “EEC”;
The letters and figures shall be 0.2 centimetres high; and
(b) for large packages, an oval mark at least 6.5 cm wide by 4.5 cm high, including the information listed under sub-paragraph (a) of this paragraph. The letters shall be at least 0.8 cm high and the figures at least 1 cm high.
2. The health mark may, in addition, include an indication enabling the official veterinary surgeon who carried out the health inspection of the meat to be identified.
3. The material used for marking shall meet all hygiene requirements and the information referred to in paragraph 1(a) above shall appear on it in perfectly legible form.
4. In the case of fresh meat produced in low throughput premises the health mark shall consist of:
(a) for meat wrapped in individual units or for small packages, a square mark, containing in legible form in letters and figures 0.2 cm high the following information:
(i)on the upper part, the letters “UK”;
(ii)in the centre, the approval number of the premises; and
(iii)on the lower part, the letter “N”; and
(b) for large packages, a square mark 5.5 cm by 5.5 cm, containing in legible form in letters 0.8 cm high and figures 1 cm high the information listed under sub-paragraph (a) of this paragraph.
5.—(a) The health mark referred to in paragraphs 1(a) and 4(a) above shall be made:
(i)on or legibly beneath wrapping or packaging of individually packed carcases;
(ii)on non-individually wrapped carcases by apposition of a seal or label, which may be used only once; or
(iii)on or legibly beneath wrapping or other packaging of parts of carcases or offal wrapped in small quantities.
(b)The health mark referred to in paragraphs 1(b) and 4(b) above shall be made on large packages containing carcases, parts of carcases or offal marked in accordance with sub-paragraph (a) of this paragraph.
(c)Where a health mark appears on the wrapping or packaging:
(i)it shall be applied in such a way that it is destroyed when the wrapping or packaging is opened; or
(ii)the wrapping or packaging shall be sealed in such a way that it cannot be re-used after opening.
6. Subject to paragraph 7 below, in cases where the poultry come from a holding which is placed under animal health restrictions in connection with a poultry disease or which is situated in an area which has been declared an avian influenza or Newcastle disease infection area, the health mark referred to in paragraph 1 above shall be—
(a) overstamped in such a way that it is covered by a diagonal cross consisting of two straight lines crossing at right angles, with the point of intersection in the centre of the stamp and the information thereon remaining legible; or
(b) replaced by a single mark, consisting of the health mark referred to in paragraph 1, overstamped in accordance with sub-paragraph (a) of this paragraph.
7.—(a) In the event of an outbreak of Newcastle disease, the health mark referred to in paragraph 1 above may be applied to poultry meat which is obtained from poultry which—
(i)comes from a holding within a surveillance zone as defined in Article 9(1) of Council Directive 92/66/EEC(7) and in respect of which no contact with an infected holding has been recorded following an epidemiological investigation;
(ii)comes from a flock in respect of which a virological examination giving a negative result has been carried out by a specified officer on a representative sample of that flock no more than five days before consignment of any of that flock;
(iii)comes from a holding where no lesions or clinical signs have been found to indicate the presence of Newcastle disease following clinical examination by a specified officer which shall be carried out not more than 24 hours before consignment of the poultry;
(iv)is transported directly from the holding of origin to the slaughterhouse using a means of transport which is sealed by a specified officer and which shall be cleaned and disinfected before and after each transportation; and
(v)is examined in the slaughterhouse at the time of the pre-slaughter health inspection or the post-mortem health inspection with a view to detect lesions or clinical signs of Newcastle disease.
(b)In this paragraph “specified officer” means a veterinary inspector appointed by the Minister under the Animal Health Act 1981
8. The health marking of carcases, parts of carcases or offal as provided for in paragraph 5(a) above shall not be necessary in the following cases:
(a) consignments of carcases, including those which have had parts removed pursuant to paragraphs 1(b) and 3(b) of Part II of Schedule 9, dispatched from a licensed slaughterhouse to a licensed cutting premises for cutting therein subject to the following conditions:
(i)the large packaging containing the fresh meat shall bear, on the external surface, the health mark in accordance with paragraphs (1)(b), 4(b) and 5(c) above;
(ii)the dispatch office shall maintain a record of the amount, type and destination of consignments dispatched in accordance with these Regulations;
(iii)the recipient cutting premises shall maintain a record of the amount, type and origin of consignments received in accordance with these Regulations;
(iv)the health mark on the large packaging shall be destroyed when the large packaging is opened in cutting premises under the supervision of the official veterinary surgeon; and
(v)the destination and intended use of the consignment shall be clearly indicated on the external surface of the large packaging in accordance with this paragraph and with Schedule 21;
(b) consignments of carcases, including those which have had parts removed pursuant to paragraph 1(b) and 3(b) of Part II of Schedule 9, parts of carcases and the following offal: hearts, livers and gizzards, dispatched from a licensed slaughterhouse, cutting premises or re-wrapping centre to a meat product establishment for preparation, subject to the following conditions:
(i)the large packaging containing the fresh poultrymeat shall bear, on the external surface, the health mark in accordance with paragraphs 1(b), 4(b) and 5(c) above;
(ii)the dispatch office shall maintain a record of the amount, type and destination of consignments dispatched in accordance with these Regulations;
(iii)the recipient meat and meat product establishment shall maintain a record of the amount, type and origin of consignments received in accordance with these Regulations;
(iv)when the fresh meat is intended for use in meat products for trade between EEA States, the health mark of the large packaging shall be destroyed when the large packaging is opened in an establishment under the supervision of the food authority or the Minister; and
(v)the destination and intended use of the consignment shall be clearly indicated on the external surface of the large packaging in accordance with this paragraph and with Schedule 21; or
(c) consignments of carcases, including those which have had parts removed pursuant to paragraph 1(b) and 3(b) of Part II of Schedule 9, dispatched from a licensed slaughterhouse, re-wrapping centre or cutting premises to restaurants, canteens and institutions for direct supply to the final consumer after heat treatment, subject to the following conditions:
(i)the packaging containing the fresh poultrymeat shall bear, on the external surface, the health mark in accordance with paragraphs 1(b), 4(b) and 5(c) above;
(ii)the dispatch office shall maintain a record of the amount, type and destination of consignments despatched in accordance with these Regulations;
(iii)the recipient outlet shall maintain a record of the amount, type and origin of consignments received in accordance with these Regulations;
(iv)those outlets shall be subject to control by the food authority, who shall be given access to the records kept; and
(v)the destination and intended use of the consignment shall be clearly indicated on the external surface of the large packaging in accordance with this paragraph and with Schedule 21.
Regulations 4(2)(a), 8(1)(d), 14(1)(g)
SCHEDULE 12STORAGE
1. After the chilling provided for in paragraph 9 of Schedule 8, fresh meat shall be kept at a temperature which may not at any time exceed +4°C.
2. Frozen meat shall be kept at a temperature which may not at any time exceed −12°C.
3. Unpackaged fresh meat shall be stored separately from packaged fresh meat.
Regulations 4(2)(a), 8(1)(d), 14(1)(h)
SCHEDULE 13WRAPPING AND PACKAGING OF FRESH MEAT
1.—(a) Packaging (for example packing cases, cardboard boxes) shall fulfil all rules of hygiene, and in particular:
(i)shall not alter the organoleptic characteristics of the meat,
(ii)shall not be capable of transmitting to the meat substances harmful to human health,
(iii)shall be strong enough to ensure effective protection of the meat during transportation and handling;
(b) packaging shall not be re-used for meat unless it is made of corrosion-resistant materials which are easy to clean and has been previously cleaned and disinfected.
2.—(a) Cut fresh meat or offal shall be wrapped immediately after cutting and in accordance with the requirements of hygiene.
(b) Wrapping shall be transparent and colourless or, in the case of coloured transparent wrapping, designed in such a way as to leave the wrapped meat or offal partially visible. It shall also fulfil the conditions of paragraph 1(a)(i) and (ii) above; it shall not be used again for wrapping meat.
(c) Parts of birds, rabbits or offal separated from the carcase shall always be wrapped in a firmly sealed protective covering satisfying the above criteria.
3. Wrapped meat shall be packaged.
4. However, when wrapping fulfils all the protective conditions of packaging it need not be transparent and colourless and placing in a second container shall not be necessary provided that the other conditions of paragraph 1 above are fulfilled.
5. Cutting, boning, wrapping and packaging operations shall not take place in the same room unless the packaging is re-usable as described in paragraph 1(b) above or where the following conditions are satisfied:
(a) the room shall be sufficiently large and so arranged that the hygiene of the operations is assured;
(b) the packaging and wrapping shall be enclosed in a sealed protective covering immediately after manufacture; this covering shall be protected from damage during transport to the licensed premises and stored under hygienic conditions in a separate room in such premises;
(c) the rooms for storing packaging material shall be dust and vermin-free and have no air connection with rooms containing substances which might contaminate fresh meat. Packaging shall not be stored on the floor;
(d) packaging shall be assembled under hygienic conditions before being brought into the room;
(e) packaging shall be hygienically brought into the room and used without delay. It shall not be handled by staff handling fresh meat;
(f) immediately after packaging the meat shall be placed in the storage room provided.
Regulations 8(1)(d), 14(1)(i)
SCHEDULE 14TRANSPORT
1. In relation to the fresh meat of birds—
(a) fresh meat shall be transported by means of transport fitted with a hermetic closing system or, in the case of such fresh meat imported from or transiting through a third country, in a sealed means of transport, designed and equipped in such a way that the temperatures specified in Schedule 12 are maintained throughout transportation;
(b) means of transport intended for transporting such fresh meat shall meet the following requirements:
(i)their inside surfaces shall be smooth and easy to clean and disinfect; and
(ii)they shall be provided with efficient devices for protecting the meat against insects and dust and be watertight;
(c) means of transport intended for transporting meat shall in no case be used for transporting live animals or any products likely to affect or contaminate meat;
(d) no other product likely to affect the hygiene of the fresh meat or to contaminate it shall be transported at the same time as the fresh meat in the same means of transport. Packaged meat shall be transported in separate means of transport from unpackaged meat unless an adequate physical separation within the same means of transport is provided so as to protect the unpackaged meat;
(e) fresh meat shall not be transported in a vehicle or container which is not clean and has not been disinfected;
(f) the occupier shall ensure that transport vehicles and loading conditions are such as to enable the hygiene requirements of this Schedule to be complied with.
2. Rabbit meat shall be dispatched in such a way that during transport it is protected from anything liable to contaminate it or to affect it unfavourably, having regard to the duration and conditions of transport and to the means of transport employed. In particular, vehicles used for such transport shall be equipped in such a way as to ensure that the temperatures laid down in paragraphs 1 and 2 of Schedule 12 are not exceeded.
Regulation 6(2)
SCHEDULE 15MEAT HYGIENE APPEALS TRIBUNAL
1. Each Tribunal shall consist of a Chairman or a Deputy Chairman and two other members.
2.—(1) The Chairman or Deputy Chairman shall be an independent person appointed by the Minister.
(2) One member shall be a person nominated by the Royal College of Veterinary Surgeons, who shall not be a member of the State Veterinary Service nor an official veterinary surgeon.
(3) One member shall be a person whom the Minister considers to be representative of the interests of licensed premises.
3. Each Tribunal may be serviced by a Secretary and such other staff as the Minister may appoint.
4. The terms of appointment and the remuneration of the members, secretary and other staff of a Tribunal shall be determined by the Minister.
Regulation 2(1)
SCHEDULE 16QUALIFICATIONS OF INSPECTORS
1. Registered Medical Practitioner.
2. Member of the Royal College of Veterinary Surgeons.
3. The holder of any of the qualifications specified in paragraph 3 of the Schedule to the Authorised Officers (Meat Inspection) Regulations 1987(8), who has undertaken a further course of training in poultry meat hygiene and inspection acceptable to the Minister.
4. The holder of a valid—
(a) Certificate in Poultry Meat Inspection of the Royal Society for the Promotion of Health; or
(b) Certificate of the former Royal Sanitary Association of Scotland; or
(c) Certificate in Poultry Meat Inspection of the Royal Environmental Health Institute of Scotland; or
(d) Certificate or other qualification in poultry meat inspection obtained in the United Kingdom or another relevant EEA State which the Minister has confirmed in writing as adequate for appointment as an inspector under these Regulations.
Regulation 12(1)(a) and Schedule 7, paragraph 1(a)
SCHEDULE 17MODEL
Regulation 15(1)
SCHEDULE 18MODEL
Regulation 15(1)
SCHEDULE 19MODEL
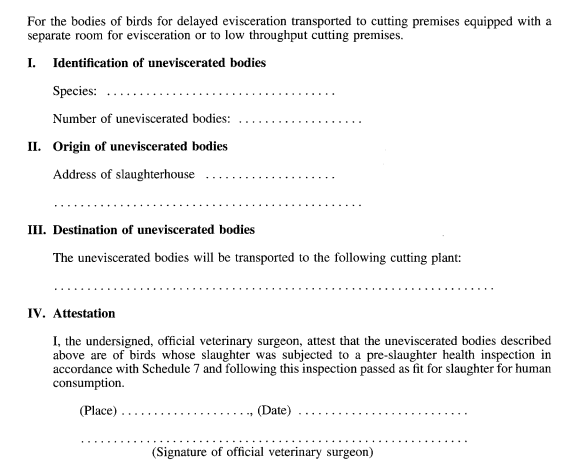
Regulation 12(5)(a) and Schedule 9, Part I, paragraph 4(b)
SCHEDULE 20MODEL
Regulations 12(2) and 14(1)(e) and Schedule 11, paragraphs 8(a)(v), (b)(v) and (c)(v)
SCHEDULE 21
Regulation 12(1)(a)
SCHEDULE 22INFORMATION TO BE SUPPLIED BY PRODUCER
PART IPRODUCTION REPORT
The production report referred to in paragraph 1(b)(ii) ofSchedule 7 shall contain at least thefollowing information:
1. Holding of origin.
2. Intended date of arrival at slaughterhouse.
3. Expected number of birds or rabbits in specified group.
4. Mortality data.
5. Details of any disease diagnosed and the results of any laboratoryexamination.
6. Details of any medication given.
PART IIADDITIONAL INFORMATION
The additional information referred to at paragraph 1(b)(iii) of Schedule 7 may consist of the following:
1. Suppliers of feeding stuffs.
2. Hatchery details.
3. Any available information on growth rates and breed performance.
4. Results of previous post-mortem inspections at the slaughterhouse onbirds or rabbits from the same specified group.
5. Feed additives and vaccines used.
Regulation 27
SCHEDULE 23AMENDMENT OF REGULATIONS
| (1) | (2) | (3) |
|---|---|---|
| References | Title | Provisions amended |
| S.I. 1960/1602 | The Food Hygiene (Docks, Carriers, etc.) Regulations 1960 | Regulation 4A(b) |
| S.I. 1966/791 | The Food Hygiene (Markets, Stalls and Delivery Vehicles) Regulations 1966 | Sub-paragraph (b) of the definition of “food business” in regulation 2 |
| S.I. 1970/1172 | The Food Hygiene (General) Regulations 1970 | Regulation 3(2)(b)(iii) |
| S.I. 1991/2825 | The Food Premises (Registration) Regulations 1991 | Regulation 3(2)(d) |
| S.I. 1992/2921 | The Meat Hygiene Appeals Tribunal (Procedure) Regulations 1992 | Regulations 1(2) and 2 |
| S.I. 1993/3247 | The Animals and Animal Products(Import and Export) Regulations 1993 | Regulation 12(2) |
| S.I. 1995/361 | The Meat (Hygiene, Inspection and Examinations for Residues) (Charges) Regulations 1995 | Paragraph (b) of Schedule 2 to the Regulations |
Options/Help
Print Options
PrintThe Whole Instrument
PrintThe Schedules only
Legislation is available in different versions:
Latest Available (revised):The latest available updated version of the legislation incorporating changes made by subsequent legislation and applied by our editorial team. Changes we have not yet applied to the text, can be found in the ‘Changes to Legislation’ area.
Original (As Enacted or Made): The original version of the legislation as it stood when it was enacted or made. No changes have been applied to the text.
More Resources
Access essential accompanying documents and information for this legislation item from this tab. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as enacted version that was used for the print copy
- lists of changes made by and/or affecting this legislation item
- confers power and blanket amendment details
- all formats of all associated documents
- correction slips
- links to related legislation and further information resources