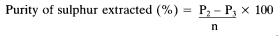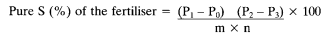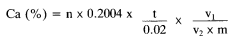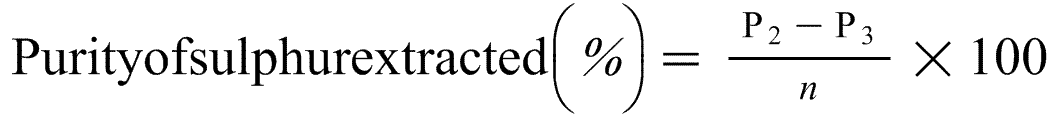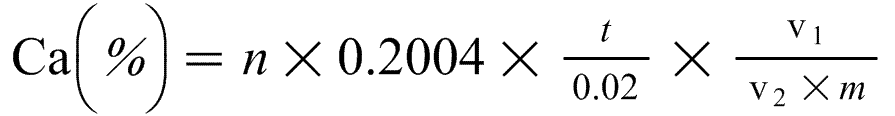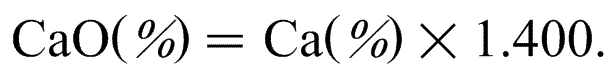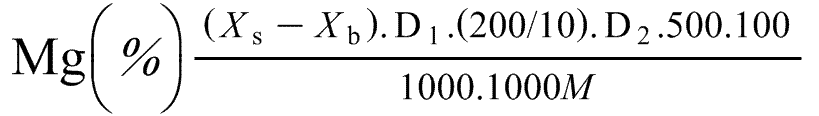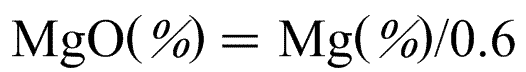- Latest available (Revised)
- Original (As made)
The Fertilisers (Sampling and Analysis) (Amendment) Regulations 1991
You are here:
- UK Statutory Instruments
- 1991 No. 2824
- Whole Instrument
- Previous
- Next
More Resources
Status:
This is the original version (as it was originally made). This item of legislation is currently only available in its original format.
Statutory Instruments
1991 No. 2824
AGRICULTURE
The Fertilisers (Sampling and Analysis) (Amendment) Regulations 1991
Made
12th December 1991
Laid before Parliament
30th December 1991
Coming into force
21st January 1992
The Minister of Agriculture, Fisheries and Food, the Secretary of State for Scotland and the Secretary of State for Wales, acting jointly, in exercise of the powers conferred on them by sections 66(1), 67(5), 74A, 75(1), 76(1), 77, 78(2), (4) and (6), 79(1), (2) and (9) and 84 of the Agriculture Act 1970(1), and of all other powers enabling them in that behalf, after consultation as required by section 84(1) of the said Act with such persons or organisations as appear to them to represent the interests concerned, hereby make the following Regulations:
Title, commencement and interpretation
1.—(1) These Regulations may be cited as the Fertilisers (Sampling and Analysis) (Amendment) Regulations 1991 and shall come into force on 21st January 1992.
(2) In these Regulations, “the principal Regulations” means the Fertilisers (Sampling and Analysis) Regulations 1991(2).
Amendment of the principal Regulations
2. The principal Regulations are hereby amended in accordance with regulations 3 and 4 below.
3.—(1) In regulation 2(3), for subparagraphs (b) and (c), there shall be substituted the following subparagraphs–
“(b)if any container holds at least 5 tonnes the prescribed amount shall be 5 tonnes; and
(c)if neither subparagraph (a) nor (b) above applies, the prescribed amount shall be the contents of the lowest number of containers together holding at least 5 tonnes.”.
(2) In regulation 2(7), for subparagraphs (b) and (c) there shall be substituted the following subparagraphs–
“(b)if any container holds at least 5,000 litres, the prescribed amount shall be 5,000 litres, and
(c)if neither subparagraph (a) nor (b) above applies, the prescribed amount shall be the contents of the lowest number of containers together holding at least 5,000 litres.”.
(3) For paragraph (2) of regulation 6 there shall be substituted the following paragraph–
“(2) Analytical constituents of materials listed in Groups 1(a), 2(a) and 3(a) of Section A, Groups 1 to 4 of Section B, Groups 1(a), 1(b) and 2 of Section C, and Section E, of the table in Schedule 1 to the Fertilisers Regulations 1991(3), shall be determined by the methods prescribed in Part I of Schedule 2.”.
4.—(1) In Part I of Schedule 2–
(a)paragraph 1(b) shall be omitted;
(b)for paragraph 2 there shall be substituted the provisions set out in Schedule 1 to these Regulations;
(c)in paragraph 3–
(i)items 12a and b, and 13a and b shall be omitted, and
(ii)after item 16g (Determination of copper) there shall be added the provisions set out in Schedule 2 to these Regulations;
(d)in the 11th line of the table for method 10, for the lines indicating nil entries in the fifth and sixth columns there shall be substituted the figures “50” and “500” respectively;
(e)the provisions relating to the following methods of analysis shall be omitted–
(i)method 12a: Determination of water-soluble magnesium — atomic absorption spectrophotometric method,
(ii)method 12b: Determination of water-soluble magnesium — EDTA method,
(iii)method 13a: Determination of total magnesium — atomic absorption spectrophotometric method, and
(iv)method 13b: Determination of total magnesium — EDTA method;
(f)in paragraph 4.3 of method 16c: Determination of Combustible Ingredients, for the expression “(See Figure 1)” there shall be substituted the expression “(See Figure 9)”;
(g)after the provisions relating to method 16g: Determination of copper, there shall be added the provisions set out in Schedule 3 to these Regulations.
(2) In Part II of Schedule 2–
(a)paragraph 1(b) shall be omitted;
(b)for paragraph 2 there shall be substituted the provisions set out in Schedule 1 to these Regulations;
(c)in paragraph 3, for item 8 there shall be substituted
“8. Determination of zinc”;
(d)for the provisions relating to method 8: Determination of total magnesium, there shall be substituted the provisions set out in Schedule 4 to these Regulations.
(3) At the end of the Appendix to Schedule 2, there shall be inserted the Diagram and key set out in Schedule 5 to these Regulations.
(4) In Schedule 3–
(a)in the first column for the results of analysis, before the line referring to “Chlorine (C)”, there shall be inserted the following provisions–
“Calcium oxide (CaO)
Total ... ... ... ...
Soluble in water ... ... ... ...
Sodium oxide (Na2O)
Total ... ... ... ...
Soluble in water ... ... ... ...
Sulphur trioxide (SO3)
Total ... ... ... ...
Soluble in water ... ... ... ...”;
(b)in the second column for the results of analysis–
(i)the expression “Magnesium (Mg)” shall be omitted, and
(ii)below the expression “Molybdenum (Mo)” there shall be added “Zinc (Zn)”.
In witness whereof the Official Seal of the Minister of Agriculture, Fisheries and Food is hereunto affixed on 10th December 1991.
L.S.
John Selwyn Gummer
Minister of Agriculture, Fisheries and Food
Strathclyde
Parliamentary Under Secretary of State, Scottish Office
9th December 1991
David Hunt
Secretary of State for Wales
12th December 1991
Regulation 4(1)(b) and 4(2)(b)
SCHEDULE 1PROVISIONS TO BE INSERTED IN PARTS I AND II OF SCHEDULE 2 TO THE PRINCIPAL REGULATIONS
“2. Reagents
Except where otherwise specified in the method of analysis, all reagents shall be of analytical quality. Where trace elements are to be determined, the purity of the reagents used shall be checked by means of a blank test.
2A. Water
(a)Except where otherwise specified, a reference in this Part of this Schedule to water shall be a reference to demineralized or distilled water.
(b)For the determination of any form of nitrogen, water shall be free of all nitrogenous compounds and carbon dioxide.
(c)Except where the method of analysis specifies a particular solvent or diluent, all dissolution, dilution, rinsing and washing operations mentioned in the methods of analysis shall be carried out using water.
2B. Apparatus
(a)Only special instruments and apparatus and specifically required apparatus and equipment are mentioned in the methods of analysis.
(b)Apparatus and equipment shall be clean.
(c)The accuracy of graduated glassware shall be assured by reference to the appropriate standards.”
Regulation 4(1)(c)(ii)
SCHEDULE 2PROVISIONS TO BE INSERTED IN PART I OF SCHEDULE 2 TO THE PRINCIPAL REGULATIONS
“17. Extraction of total calcium, total magnesium, total sodium and total sulphur in the form of sulphates
18. Extraction of total sulphur
19. Extraction of water-soluble calcium, magnesium, sodium and sulphur (in the form of sulphates)
20. Extraction of water-soluble sulphur
21. Extraction and determination of elemental sulphur
22. Manganimetric determination of extracted calcium following precipitation in the form of oxalate
23. Determination of magnesium by atomic absorption spectrophotometry
24. Determination of magnesium by complexometry
25. Determination of sulphates
26. Determination of the sodium extracted”
Regulation 4(1)(g)
SCHEDULE 3PROVISIONS TO BE ADDED TO PART I OF SCHEDULE 2 TO THE PRINCIPAL REGULATIONS
“17. EXTRACTION OF TOTAL CALCIUM, TOTAL MAGNESIUM, TOTAL SODIUM AND TOTAL SULPHUR IN THE FORM OF SULPHATES
1. Scope
This method is for the extraction of total calcium, total magnesium, total sodium and total sulphur present in the form of sulphates.
2. Field of application
This method applies to all fertilisers, for which a declaration of the total calcium, total magnesium, total sodium and total sulphur in the form of sulphates is required.
3. Principle
Dissolution by boiling in dilute hydrochloric acid.
4. Reagents
4.1 Diluted hydrochloric acid:
One volume of hydrochloric acid (d = 1.18 g/ml) plus one volume of water.
5. Apparatus
Electric hot plate with adjustable temperature.
6. Preparation of the sample
See method 1.
7. Procedure
7.1 Test sample.
Calcium, magnesium, sodium and sulphur in the form of sulphates are extracted from a test sample of five grams weighed to within one milligram.
However, when the fertiliser contains more than 15% of sulphur (S) i.e. 37.5% SO3, and more than 18.8% of calcium (Ca) i.e. 26.3% CaO, the extraction of calcium and sulphur is carried out on a test sample of one gram, weighed to within one milligram. Place the test sample in a 600 millilitre beaker.
7.2 Preparation of the solution.
Add approximately 400 millilitres of water and, taking care when the sample contains a significant quantity of carbonates, 50 millilitres of dilute hydrochloric acid (4.1) a small amount at a time. Bring to the boil and maintain for 30 minutes. Allow to cool, stirring occasionally. Transfer quantitatively into a 500 millilitre graduated flask. Make up to volume with water, and mix. Pass through a dry filter into a dry container, discarding the initial portion. The extract must be completely clear. Stopper if the filtrate is not used immediately.
18. EXTRACTION OF TOTAL SULPHUR
1. Scope
This method is for the extraction of total sulphur contained in fertilisers in elemental form and/or in other chemical combinations.
2. Field of application
This method applies to all fertilisers for which a declaration of the total sulphur present in various forms (elemental, thiosulphate, sulphite, sulphate) is required.
3. Principle
Elemental sulphur is converted in an alkaline medium into polysulphides and thiosulphate; these, together with any sulphites which may be present, are then oxidized with hydrogen peroxide. The various forms of sulphur are thus converted into sulphate which is determined by precipitation as barium sulphate (method 25).
4. Reagents
4.1 Diluted hydrochloric acid:
One volume of hydrochloric acid (d = 1.18 g/ml) plus one volume of water.
4.2 Sodium hydroxide solution, NaOH, 30% minimum (d = 1.33).
4.3 Hydrogen peroxide solution, 30% w/w.
4.4 Aqueous solution of barium chloride BaC122H20, 122 gram per litre.
5. Apparatus
Electric hot plate with adjustable temperature.
6. Preparation of the sample
See method 1.
7. Procedure
7.1 Test sample.
Weigh out to within one milligram a quantity of fertiliser containing between 80 and 350 milligrams of sulphur (S) or 200 and 875 milligrams SO3.
As a rule (Where S < 15%), weigh out 2.5 grams. Place the test sample in a 400 millilitre beaker.
7.2 Oxidation.
Add 20 millilitres of sodium hydroxide solution (4.2) and 20 millilitres of water. Cover with a watch glass. Boil for five minutes on the hot plate (5). Remove from the hot plate. Using a jet of hot water, collect any material sticking to the sides of the beaker and boil for 20 minutes. Leave to cool.
Add 2 millilitre increments of hydrogen peroxide (4.3) until no reaction is observed. Six to eight millilitres of hydrogen peroxide will be necessary. Allow oxidation to continue for one hour, then bring to the boil for half an hour. Leave to cool.
7.3 Preparation of the solution to be analysed.
Add approximately 50 millilitres of water and 50 millilitres of the hydrochloric acid solution (4.1).
If the level of sulphur (S) is less than 5%:
filter into a 600 millilitre beaker. Wash the residue on the filter several times with cold water. After washing, check for the absence of sulphate in the last drops of the filtrate using the barium chloride solution (4.4). The filtrate must be perfectly clear. Sulphate is determined on the whole of the filtrate in accordance with method 25.
If the level of sulphur (S) is at/above 5%:
transfer quantitatively into a 250 millilitre volumetric flask, make up to volume with water and mix. Filter through a dry filter into a dry container; the filtrate must be completely clear. Stopper if the solution is not to be used immediately. Determine sulphates on an aliquot of this solution by precipitation in the form of barium sulphate (method 25).
19. EXTRACTION OF WATER-SOLUBLE CALCIUM, MAGNESIUM, SODIUM AND SULPHUR (IN THE FORM OF SULPHATES)
1. Scope
This method is for the extraction of water-soluble calcium, magnesium, sodium and sulphur (in the form of sulphates), so that the same extract can be used to determine each nutrient required.
2. Field of application
This method applies solely to fertilisers for which a declaration of the water-soluble calcium, magnesium, sodium and sulphur (in the form of sulphates) is required.
3. Principle
The nutrients are dissolved in boiling water.
4. Reagents
Distilled or demineralized water of equivalent quality.
5. Apparatus
Electric hot plate with adjustable temperature.
6. Preparation of the sample
See method 1.
7. Procedure
7.1 Test sample.
(a)Where fertilisers contain no sulphur or where they contain, at the same time, no more than 3% of sulphur (S) i.e. 7.5% SO3, and no more than 4% of calcium (Ca) i.e. 5.6% CaO, weigh out five grams of fertiliser to within one milligram.
(b)Where fertilisers contain more than 3% of sulphur (S) and more than 4% of calcium (Ca), weigh out one gram of fertiliser to within one milligram.Place the test sample in a 600 millilitre beaker.
7.2 Preparation of the solution.
Add approximately 400 millilitres of water and boil for 30 minutes. Allow to cool, stirring occasionally, and transfer quantitatively into a 500 millilitre graduated flask. Make up to volume with water and mix.
Filter through a dry filter into a dry container: Discard the initial portion of the filtrate. The filtrate must be completely clear.
Stopper if the solution is not to be used immediately.
20. EXTRACTION OF WATER-SOLUBLE SULPHUR
1. Scope
This method is for the extraction of water-soluble sulphur contained in fertilisers, in various forms.
2. Field of application
This method applies to all fertilisers for which a declaration of the water-soluble sulphur trioxide is required.
3. Principle
Soluble forms of sulphur are dissolved in cold water and converted into sulphate by oxidation with hydrogen peroxide in an alkaline medium.
4. Reagents
4.1 Diluted hydrochloric acid:
One volume of hydrochloric acid (d = 1.18 g/ml) plus one volume of water.
4.2 Sodium hydroxide solution containing at least 30% NaOH (d = 1.33g/ml).
4.3 Hydrogen peroxide solution, 30% w/w.
5. Apparatus
5.1 500 millilitre graduated Stohmann flask.
5.2 Rotary shaker, 30 to 40 turns per minute.
5.3 Electric hot plate with adjustable temperature.
6. Preparation of the sample
See method 1.
7. Procedure
7.1 Test sample.
(a)Where fertilisers contain a maximum of 3% of sulphur (S) i.e. 7.5% SO3, together with a maximum of 4% of calcium (Ca) i.e. 5.6% CaO, weigh out 5 grams of fertiliser to within one milligram.
(b)Where fertilisers contain more than 3% of sulphur (S) together with more than 4% of calcium (Ca), weigh out one gram of fertiliser to within one milligram.
Place the test sample in a 500 millilitre flask (5.1).
7.2 Preparation of the solution.
Add approximately 400 millilitres of water. Stopper. Shake (5.2) for 30 minutes. Make up to volume with water and mix. Pass through a dry filter into a dry container. Reject the first portion of the filtrate. Stopper if the solution is not to be used immediately.
7.3 Oxidation of the aliquot portion to be analysed.
Take an aliquot of the extraction solution not exceeding 50 millilitres and, if possible, containing between 20 and 100 milligrams of sulphur (S).
Make up the volume to 50 millilitres with water, if necessary. Add three millilitres of sodium hydroxide solution (4.2) and two millilitres of hydrogen peroxide solution (4.3). Cover with a watch glass and boil gently for one hour on the hot plate (5.3). Keep adding one millilitre increments of hydrogen peroxide solution for as long as the reaction continues (maximum quantity five millilitres).
Then leave to cool. Remove the watch glass and wash the underside into the beaker. Add approximately 20 millilitres of dilute hydrochloric acid (4.1). Make up to approximately 300 millilitres with water.
Determine the content of sulphates on the whole of the oxidised solution in accordance with method 25.
21. EXTRACTION AND DETERMINATION OF ELEMENTAL SULPHUR
WARNING
This method of analysis involves the use of carbon disulphide (CS2). Special safety measures must therefore be taken, in particular with regard to:
the storage of CS2,
protective equipment for staff,
occupational hygiene,
prevention of fires and explosions,
disposal of the reagent.
This method requires highly skilled staff and a suitably equipped laboratory.
1. Scope
This method is for the extraction and determination of the elemental sulphur content of fertilisers.
2. Field of application
This method applies to all fertilisers for which a declaration of the total sulphur in elemental form is required.
3. Principle
After the removal of soluble compounds, elemental sulphur is extracted by using carbon disulphide, followed by gravimetric determination of the sulphur extracted.
4. Reagents
Carbon disulphide.
5. Apparatus
5.1 100 millilitre extraction flask with ground glass stopper.
5.2 Soxhlet apparatus, with the appropriate filter elements.
5.3 Vacuum rotary evaporator.
5.4 Electric oven, fan assisted, set at 90 ± 2°C.
5.5 Petri dishes, five to seven centimetres in diameter, not exceeding five centimetres in height.
5.6 Electric hot plate with adjustable temperature.
6. Preparation of the sample
See method 1.
7. Procedure
7.1 Test sample.
Weigh out five to ten grams of the sample to an accuracy of one milligram and place in the thimble of the Soxhlet apparatus (5.2).
7.2 Extraction of the sulphur.
Wash thoroughly the contents of the thimble with hot water to remove all soluble compounds. Dry in the oven at 90°C (5.4) for at least one hour. Place the thimble in the Soxhlet apparatus (5.2).
Place a few glass beads in the flask of the apparatus (5.1) and weigh (P0), then add 50 millilitres of carbon disulphide (4.1).
Connect the apparatus, switch on and leave the elemental sulphur to be extracted for six hours. Switch off the heat and, after cooling, disconnect the flask. Connect the flask to the rotary evaporator (5.3) and evaporate until the contents of the flask have solidified in a spongy mass.
8. Expression of results
The percentage elemental sulphur (S) content of the fertiliser is as follows:
Where:
m = the mass of the test sample of fertiliser in grams,
P0 = the mass of the Soxhlet flask in grams,
P1 = the mass of the Soxhlet flask and the impure sulphur after drying,
n = the mass of the impure sulphur to be purified in grams,
P2 = the mass of the Petri dish and the sample,
P3 = the mass of the Petri dish after sublimation of the sulphur.
22. MANGANIMETRIC DETERMINATION OF EXTRACTED CALCIUM FOLLOW-ING PRECIPITATION IN THE FORM OF OXALATE
1. Scope
This method is for the determination of calcium in fertiliser extracts.
2. Field of application
This method applies to all fertilisers for which a declaration of the total and/or water-soluble calcium is required.
3. Principle
Precipitation of the calcium contained in an aliquot of the extraction solution in the form of an oxalate, which is determined by titration using potassium permanganate.
4. Reagents
4.1 Diluted hydrochloric acid:
One volume of hydrochloric acid (d = 1.18 g/ml) plus one volume of water.
4.2 1:10 dilute sulphuric acid:
One volume of sulphuric acid (d = 1.84 g/ml) in ten volumes of water.
4.3 1:1 dilute ammonia solution:
One volume of ammonia (d = 0.88 g/ml) and one volume of water.
4.4 Saturated solution of ammonium oxalate [(NH4)2 C2O4 H2O] at ambient temperature (approximately 40 grams per litre).
4.5 Citric acid solution, 30% (m/v).
4.6 Ammonium chloride solution, 5% (m/v).
4.7 Solution of bromothymol blue in 95% ethanol, 0.1% (m/v).
4.8 Solution of bromocresol green in 95% ethanol 0.04% (m/v).
4.9 Standard solution of potassium permanganate, 0.02 M.
5. Apparatus
5.1 Filter crucible with 5 to 20 μ porosity sintered glass.
5.2 Hot water bath.
6. Preparation of the aliquot to be analysed
Using a pipette, take an aliquot portion of the extraction solution obtained by method 17 or 19, containing between 15 and 50 milligrams of Ca (= 21 to 70 milligrams of CaO). Let the volume of this aliquot be v2. Pour into a 400 millilitre beaker. If necessary, neutralise (change of indicator (4.7) from green to blue) with a few drops of the ammonia solution (4.3).
And one millilitre of the citric acid solution (4.5) and five millilitres of ammonium chloride solution (4.6).
7. Precipitation of the calcium oxalate
Add approximately 100 millilitres of water. Bring to boil, add 8 to 10 drops of indicator solution (4.8) and, slowly, 50 millilitres of a hot ammonium oxalate solution (4.4), stirring constantly. If a precipitate forms, dissolve by adding a few drops of hydrochloric acid (4.1). Neutralise very slowly with ammonia solution (4.3) while stirring continuously to a pH of 4.4 to 4.6 (change of indicator (4.8) from green to blue). Place the beaker in a boiling hot water bath (5.2) for approximately 30 minutes.
Remove the beaker from the bath, leave standing for an hour and filter through the crucible (5.1).
8. Titration of the oxalate precipitate
Wash the beaker and crucible until the excess ammonium oxalate has been completely removed (this can be checked by the absence of chloride in the washing water). Place the crucible in the 400 millilitre beaker and dissolve the precipitate with 50 millilitres of hot sulphuric acid (4.2). Add water to the beaker in order to obtain a volume of approximately 100 millilitres. Bring to a temperature of 70 to 80°C and titrate drop by drop with the permanganate solution (4.9) until the pink colour lasts for a minute. Let this volume be n.
9. Expression of results
The calcium (Ca) content of the fertiliser is as follows:
Where:
n = the volume of 0.2M permanganate used, in millilitres,
m = the mass of the test sample in grams,
v2 = the aliquot volume in millilitres,
v1 = the volume of the extraction solution in millilitres,
t = the molarity of the permanganate solution in moles per litre.
CaO (%) = Ca (%) × 1.400.
23. DETERMINATION OF MAGNESIUM BY ATOMIC ABSORPTION SPEC-TROPHOTOMETRY
1. Scope
This method is for the determination of magnesium in fertiliser extracts.
2. Field of application
This method applies to all fertiliser extracts obtained by methods 17 and 19, for which a declaration of the total magnesium and/or water-soluble magnesium is required, with the exception of kieserite, magnesium sulphate, magnesium chloride solution and kieserite with potassium sulphate to which method 24 applies.
The method set out below applies to all fertiliser extracts containing elements in quantities that might interfere with the complexometric determination of magnesium.
3. Principle
Determination of magnesium by atomic absorption spectrophotometry after appropriate dilution of the extract.
4. Reagents
4.1 Hydrochloric acid, 1M solution.
4.2 Hydrochloric acid, 0.5M solution.
4.3 Standard solution of magnesium, 1.00 milligrams per millilitre.
4.3.1 Dissolve 1.013 grams of magnesium sulphate (MgSO4,7H2O) in 0.5M hydrochloric acid solution (4.2).or:
4.3.2 weigh out 1.658 grams of magnesium oxide (MgO), previously calcined to remove all traces of carbonation. Place in a beaker with 100 millilitres of water and 120 millilitres of 1M hydrochloric acid (4.1). When it has dissolved, transfer quantitatively into a 1000 millilitre graduated flask. Make up to the volume and mix.
or:
4.3.3 Commercial standard solution.
The laboratory is responsible for testing such solutions.
4.4 Strontium chloride solution.
Dissolve 75 grams of strontium chloride (SrCL2 6H2O) in the hydrochloric acid solution (4.2) and make up to 500 millilitres with the same acid solution.
5. Apparatus
5.1 Spectrophotometer fitted for atomic absorption, with a magnesium lamp, set at 285.2 nm.
5.2 Air-acetylene flame.
6. Preparation of the solution to be analysed
See methods 17 and 19.
7. Procedure
7.1 If the fertiliser has a declared magnesium (Mg) content of more than 6&7percnt; (i.e. 10% as MgO), take 25 millilitres (V1) of the extraction solution (6). Transfer into a 100 millilitre graduated flask, and make up to volume with water and mix. The solution factor is D1 = 100/V1.
7.2 Using a pipette, take 10 millilitres of the extraction solution (6) or the solution (7.1). Transfer into a 200 millilitre graduated flask. Make up to volume with the 0.5 M hydrochloric acid solution (4.2) and mix. The dilution factor is 200/10.
7.3 Dilute this solution (7.2) with the 0.5M hydrochloric acid solution (4.2) so as to obtain a concentration in the optimum working field of the spectrophotometer (5.1). V2 is the volume of the sample in 100 millilitres. The dilution factor is D2 = 100/V2.
The final solution should contain 10% v/v of the strontium chloride solution (4.4).
7.4 Preparation of blank solution.
Prepare a blank solution by repeating the whole procedure from the extraction (method 17 or 19), omitting only the test sample of fertiliser.
7.5 Preparation of calibration solutions.
By diluting the standard solution (4.3) with 0.5M hydrochloric acid, prepare at least five calibration solutions of increasing concentration within the optimum measuring range of the apparatus (5.1).
These solutions should contain 10% v/v of the strontium chloride solution (4.4).
7.6 Measurement.
Set up the spectrophotometer (5.1) at a wavelength of 285.2 nm.
Spray, successively, the calibration solutions (7.5), the sample solution (7.3) and the blank solution (7.4), washing the instrument through with the solution to be measured next. Repeat this operation three times. Plot the calibration curve using the mean absorbances of each of the calibration solutions (7.5) as the ordinates and the corresponding concentration of magnesium in μg/ml as the abscissae. Determine the conentration of magnesium in the sample (7.3), Xs and blank (7.4), Xb, by reference to the calibration curve.
8. Expression of results
Calculate the amount of magnesium (Mg) or magnesium oxide (MgO) in the sample by reference to the calibration solutions and taking into consideration the blank.
The percentage of magnesium (Mg) in the fertiliser is equal to:
Where:
Xs = the concentration of the solution as calculated from the calibration curve, in μg/ml.
Xb = the concentration of the blank solution as calculated from the calibration curve, in μg/ml.
D1 = the dilution factor if the solution is diluted (7.1).
It is equal to four if 25 millilitres are taken.
It is equal to one when the solution is not diluted.
D2 = the dilution factor in 7.3.
M = the mass of the test sample taken for the extraction.
MgO (%) = Mg (%)/0.6
24. DETERMINATION OF MAGNESIUM BY COMPLEXOMETRY
1. Scope
This method is for the determination of magnesium in fertiliser extracts.
2. Field of application
This method applies to the determination of total magnesium and/or water-soluble magnesium in the following fertilisers:
straight nitrogenous fertilisers (calcium magnesium nitrate, magnesium sulphoni-trate, nitrogenous fertilisers with magnesium) and straight potassic fertilisers (enriched kainite, potassium chloride containing magnesium, potassium sulphate containing magnesium salt), kieserite, magnesium sulphate, magnesium chloride solution, and kieserite with potassium sulphate.
3. Principle
The magnesium is extracted by methods 17 and/or 19. First titration: with EDTA and Ca and Mg in the presence of Eriochrome black T. Second titration: with EDTA of Ca in the presence of calcein or of calcon carbonic acid. Determination of magnesium by difference.
4. Reagents
4.1 Standard 0.05M solution of magnesium:
4.1.1 Dissolve 1.232 grams of magnesium sulphate (MgSO4 7H20) in the 0.5 M hydrochloric acid solution (4.11) and make up to 100 millilitres with the same acid.
or:
4.1.2 Weigh out 2.016 grams of magnesium oxide, previously ashed to remove all traces of carbonates. Place it in a beaker with 100 millilitres of water.
Stir in approximately 120 millilitres of approximately 1M hydrochloric acid (4.12).
After dissolution, transfer quantitatively into a graduated 1000 millilitre flask. Make up to volume and mix.
One millilitre of these solutions should contain 1.216 milligrams of Mg (= 2.016 milligrams of MgO).
The laboratory is responsible for testing the strength of this standard solution.
4.2 0.05M solution of EDTA.
Weigh out 18.61 grams of the dihydrated disodium salt of ethylenediaminetet-raacetic (C1and0H1and4N2Na2O82H2O), place it in a 1000 millilitre beaker and dissolve in 600 to 800 millilitres of water. Transfer the solution quantitatively into a graduated 1000 millilitre flask. Make up the volume and mix. Check this solution with the standard solution (4.1) by taking a sample of 20 millilitres of the latter and by titration according to the analytical procedure described at 7.2.
One millilitre of the EDTA solution should correspond to 1.216 milligrams of Mg (= 2.016 milligrams of MgO) and to 2.004 milligrams of Ca (= 2.804 milligrams Ca2) (see remarks 10.1 and 10.6).
4.3 0.05 molar standard solution of calcium.
Weigh out 5.004 grams of dry calcium carbonate. Place it in a beaker with 100 millilitres of water. Progressively stir in 120 millilitres of approximately 1M hydrochloric acid (4.12).
Bring to the boil in order to drive off the carbon dioxide, cool, transfer quantitatively into a graduated one-litre flask, make up the volume with water and mix. Check this solution against the EDTA solution (4.2) following analytical procedure (8.3). One millilitre of this solution should contain 2.004 milligrams of Ca (= 2.804 milligrams of CaO) and should correspond to one millilitre of the 0.05M EDTA solution (4.2).
4.4 Calcein indicator.
Carefully mix in a mortar one gram of calcein with 100 grams of sodium chloride. Use 10 milligrams of this mixture. The indicator changes colour from green to orange. Titration must be carried out until an orange colour free from green tinges is obtained.
4.5 Calcon carbonic acid indicator.
Dissolve 400 milligrams of calcon carbonic acid in 100 millilitres of methanol. This solution may only be kept for approximately four weeks. Use three drops of this solution. The indicator changes colour from red to blue. Titration must be carried out until a blue colour free from red tinges is obtained.
4.6 Eriochrome black-T indicator.
Dissolve 300 milligrams of Eriochrome black-T in a mixture of 25 millilitres of propan-1-ol and 15 millilitres of triethanolamine. This solution may only be kept for approximately four weeks. Use three drops of this solution. This indicator changes colour from red to blue and titration must be carried out until a blue colour free from red tinges is obtained. It changes colour only when magnesium is present. If necessary add one millilitre of the standard solution (4.1).
When both calcium and magnesium are present the EDTA first forms a complex with the calcium and then with the magnesium. In that case the two elements are determined concurrently.
4.7 Potassium cyanide solution.
Aqueous solution of KCN at 2%. (Do not pipette by mouth and see 10.7.)
4.8 Solution of potassium hydroxide and potassium cyanide.
Dissolve 280 grams of KOH and 66 grams of KCN in water, make up the volume to one litre and mix.
4.9 pH 10.5 buffer solution.
In a 500 millilitre graduated flask, dissolve 33 grams of ammonium chloride in 200 millilitres of water, add 250 millilitres of ammonia (d = 0.91) make up the volume with water and mix. Check the pH of the solution regularly.
4.10 Diluted hydrochloric acid:
One volume of hydrochloric acid (d = 1.18 g/ml) plus one volume of water.
4.11 Hydrochloric acid solution approximately 0.5M.
4.12 Hydrochloric acid solution approximately 1M.
4.13 Sodium hydroxide solution 5 M.
5. Apparatus
5.1 Magnetic or mechanical stirrer.
5.2 pH meter.
6. Control test
Carry out a determination on aliquots of solutions (4.1 and 4.3) such that the Ca/Mg ratio is approximately equal to that of the solutions to be analysed. To this end take (a) of standard solution (4.3) and (b-a) of standard solution (4.1). (a) and (b) are the volumes of EDTA solution in millilitres used in the two titrations performed on the solution to be analysed. This procedure is correct only if the solutions of EDTA, calcium and magnesium are exactly equivalent. If this is not the case, it is necessary to make corrections.
7. Preparation of the solution to be analysed
See methods 17 and 19.
8. Determination
8.1 Aliquot samples to be taken.
This aliquot will as far as possible contain between 9 and 18 milligrams of magnesium (= 15 to 30 milligrams of MgO).
8.2 Titration in the presence of Eriochrome black-T.
Pipette an aliquot (8.1) of the solution to be analysed into a 400 millilitre beaker. Neutralize the excess acid with the 5M sodium hydroxide solution (4.12) and check the pH. Dilute with water to approximately 100 millilitres. Add 5 millilitres of the buffer solution (4.9). The pH measured by the meter must be 10.5 ± 0.1. Add 2 millilitres of the potassium cyanide solution (4.7) and three drops of the Eriochrome black-T indicator (4.6). Titrate with the EDTA solution (4.2). Stirring gently with the stirrer (5.1) (see 10.2, 10.3 and 10.4). Let “b” be the volume in millilitres of 0.05 molar EDTA solution used.
8.3 Titration in the presence of calcein or of calcon carbonic acid.
Pipette an aliquot of the solution to be analysed equal to that taken for the above titration and place it in a 400 millilitre beaker. Neutralise the excess acid with the 5M sodium hydroxide solution (4.13) using the pH meter. Dilute with water to about 100 millilitres. Add 10 millilitres of the KOH/KCN solution (4.8) and three drops of the indicator (4.4 or 4.5). Stirring gently with the stirrer (5.1) titrate with the EDTA solution (4.2) (see 10.2, 10.3 and 10.4). Let “a” be the volume in millilitres of 0.05M EDTA solution.
9. Expression of results
For the EEC fertilisers to which the method is applicable (5 grams of fertiliser in 500 millilitres of extract), the percentage content of the fertiliser is:
Where:
a = the volume in millilitres of 0.05M EDTA solution used for the titration in the presence of calcein or calcon carbonic acid.
b = the volume in millilitres of 0.05M EDTA solution used for the titration in the presence of Eriochrome black-T.
M = the mass of the sample present in the aliquot taken (in grams).
T = 0.2016 × molarity of the EDTA solution/0.05 (see 4.2).
T1 = 0.1216 × molarity of the EDTA solution/0.05 (see 4.2).
10. Remarks
10.1 The stoichiometric EDTA-metal ratio in the complexometric analyses is always 1:1 whatever the valency of the metal and in spite of the fact that EDTA is quadrivalent. The EDTA titration solution and the standard solutions will therefore be molar and not normal.
10.2 Complexometric indicators are often sensitive to air. The solution may lose colour during titration. In this case, one or two additional drops of indicator must be added. This is true particularly in the case of Eriochrome black-T and calcon carbonic acid.
10.3 The metal-indicator complexes are often relatively stable and it may take some time for the colour to change. The last drops of EDTA must therefore be added slowly and a drop of 0.05 molar solution of magnesium (4.1) or calcium (4.3) added to ensure that the colour change has not already taken place. This is particularly true in the case of the eriochrome-magnesium complex.
10.4 The colour change of the indicator must not be observed vertically, but horizontally across the solution and the beaker must be placed against a white background in a well-lit position. The colour change of the indicator may also be observed easily by placing the beaker on frosted glass lit moderately from below (25 watt lamp).
10.5 This analysis requires a certain amount of experience. The task will involve, among other things, observing the colour changes of standard solutions 4.1 and 4.3. It is recommended that the determinations be carried out by the same laboratory chemist.
10.6 If an EDTA solution of guaranteed strength is used (Titrisol, Normex, for example) this may simplify the control of the equivalence of standard solutions 4.1, 4.2 and 4.3.
10.7 The solutions containing potassium cyanide must not be poured down the sink until the cyanide has been converted into a harmless compound, for example, by oxidation with sodium hypochlorite after having been made alkaline.
25. DETERMINATION OF SULPHATES
1. Scope
This method is for the determination of sulphur present in fertiliser extracts in the form of sulphates.
2. Field of application
This method applies to the determination of sulphates present in the extractions performed in methods 17, 18, 19 and 20.
3. Principle
Gravimetric determination as barium sulphate.
4. Reagents
4.1 Diluted hydrochloric acid:
One volume of hydrochloric acid (d = 1.18 g/ml) and one volume of water.
4.2 Barium chloride solution BaC12. 2H2O:122 grams per litre.
4.3 Silver nitrate solution: 5 grams per litre.
5. Apparatus
5.1 Crucibles.
5.2 Hot water bath.
5.3 Drying oven set at 105°C ± 1°C.
5.4 Electric furnace set at 800°C ± 50°C.
6. Procedure
6.1 Sampling of the solution.
Pipette an aliquot of one of the extraction solutions indicated at 2 containing between 20 and 100 milligrams of S or 50 and 250 milligrams of SO3.
Place this aliquot in a beaker of suitable capacity. Add 20 millilitres of dilute hydrochloric acid (4.1). Make up to about 300 millilitres with water.
6.2 Preparation of the precipitate.
Bring the solution to the boil. Add, drop by drop, about 20 millilitres of the hot barium chloride solution (4.2) while stirring the solution vigorously. Boil for a few minutes.
Place the beaker, covered with a watch glass, in a boiling water bath (5.2) for an hour. Then leave standing hot (~60°C) until the supernatant liquor is clear. Decant the clear solution through a slow filtration ash-free filter. Wash the precipitate several times with hot water. Continue to wash the precipitate on the filter until the filtrate is chloride free. This can be checked by using silver nitrate solution (4.3).
6.3 Incineration and weighing of the precipitate.
Place the filter paper and precipitate in a crucible (5.1) previously weighed to the nearest 0.1 milligrams. Dry in the oven (5.3) and ash at approximately 800°C for half an hour (5.4). Allow to cool in a desiccator and weigh to within 0.1 milligrams.
7. Expression of results
One milligram of barium sulphate corresponds to 0.137 milligrams of S or to 0.343 milligrams of SO3.
The percentage S content of the fertiliser is obtained as follows:
Where:
w = the mass of the barium sulphate precipitate in milligrams,
v1 = the volume of the extraction solution in millilitres,
v2 = the aliquot volume in millilitres,
m = the mass of the test sample in grams.
26. DETERMINATION OF THE SODIUM EXTRACTED
1. Scope
This method is for the determination of sodium in fertiliser extracts.
2. Field of application
This method applies to fertilisers for which a declaration of sodium is required.
3. Principle
Following suitable dilution of the extract obtained via method 17 and/or 19 the sodium content of the solution is determined by flame-emission spectrophotometry.
4. Reagents
4.1 Diluted hydrochloric acid:
One volume of hydrochloric acid (d = 1.18 g/ml) plus one volume of water.
4.2 Aluminium nitrate Al(NO3)3,9H2O.
4.3 Caesium chloride, CsCl.
4.4 Anhydrous sodium chloride, NaCl.
4.5 Caesium chloride and aluminium nitrate solution.
Dissolve in water 50 grams of caesium chloride (4.3) and 250 grams of aluminium nitrate (4.2) in a 1000 millilitre graduated flask. Make up to volume with water and mix.
4.6 Standard sodium solution of one milligram/millilitre of Na.
Dissolve in water 2.542 grams of sodium chloride (4.4) in a 1000 millilitre graduated flask. Add 10 millilitres of hydrochloric acid (4.1). Make up to volume with water and mix.
5. Apparatus
Spectrophotometer equipped for flame emission, set at 589.3 nm.
6. Calibration solutions
6.1 Pipette 10 millilitres of standard solution (4.6) into a 250 millilitre graduated flask. Make up to volume and mix. Concentration of solution: 40 μg/ml of Na.
6.2 Using a burette place 0, 5, 10, 15, 20, 25 millilitres of the intermediate solution (6.1) in 100 milliltres graduated flasks. Add 10 millilitres of the solution (4.5). Make up to volume and mix. Concentration of solutions: 0, 2, 4, 6, 8, 10 μg/ml of Na.
7. Preparation of solutions to be measured
Depending upon the expected sodium content of the extraction solution as in method 17 or 19 (five grams of fertiliser in 500 millilitres), carry out the dilutions in accordance with the following table:
| Na2O (%) | Na (%) | |||||
|---|---|---|---|---|---|---|
| Intermediate dilution | ||||||
| Sample (ml) (v2) | Dilution to ml (v3) | |||||
| Final dilution | ||||||
| Sample (ml) (v4) | Dilution to ml | |||||
| Degree of dilution | ||||||
| 3–5 | 2.2–3.7 | 10 | 50 | 10 | 100 | 50 |
| 5–10 | 3.7–7.4 | 10 | 100 | 10 | 100 | 100 |
| 10–20 | 7.4–15 | 10 | 100 | 5 | 100 | 200 |
| 20–38 | 15–28 | 5 | 100 | 5 | 100 | 400 |
Make up the intermediate dilution with water. For the final dilution add ten millilitres of the solution (4.5) to the 100 millilitre graduated flask.
For a test sample of one gram multiply the volume of the final dilution (V4) by five.
8. Determination
Prepare the spectrophotometer (5) for the measurements at 589.3 nm. Calibrate the instrument by measuring the response of the calibration solutions (6.2). Then adjust the sensitivity of the instrument to use its entire scale when the most concentrated calibration solution is used. Then measure the response of the sample solution to be analysed (7). Repeat this operation twice.
9. Calculation of results
Draw a calibration curve by plotting the average response for each calibration solution along the ordinate and the corresponding concentrations, expressed in μg per millilitre on the abscissa. Determine from this the sodium concentration of the test solution. Calculate the quantity of sodium from the standard solutions taking account of the levels of dilution. Express the results as a percentage of the sample.
The percentage sodium (Na) content of the fertiliser is as follows:
Where:
x = the concentration of the solution introduced into the spectrophotometer in μg/ml,
v1 = the volume of the extraction solution in millilitres,
v2 = the aliquot volume in the intermediate dilution in millilitres,
v3 = the volume of intermediate dilution in millilitres,
v4 = the aliquot volume in ml of the final dilution (in 100 millilitres),
m = the mass of the test sample in grams.”
Regulation 4(2)(d)
SCHEDULE 4PROVISIONS TO BE INSERTED IN PART II OF SCHEDULE 2 TO THE PRINCIPAL REGULATIONS
8. DETERMINATION OF ZINC
1. Scope and field of application
This method is for the determination of zinc.
2. Principle
The sample is dissolved directly in hydrochloric acid. The element is determined by atomic absorption spectrophotometry.
3. Reagents
3.1 Hydrochloric acid (d = 1.18g/ml).
3.2 Hydrochloric acid solution, 6N.
3.3 Hydrochloric acid solution, 0.5N.
3.4 Zinc solution (stock): weigh to the nearest 0.001g, 1g pure zinc, dissolve in 25ml 6N hydrochloric acid (3.2) and make up to 1,000ml with water. 7ml of this solution = 1000 μg zinc (Zn).
3.4.1 Zinc solution (dilute): dilute 10.0ml of stock solution (3.4) to 1,000ml with water.
1 ml of this solution = 10 μg zinc (Zn).
NOTE:commercially prepared standard solutions of zinc may also be used.
3.5 Lanthanum chloride solution: dissolve 12g lanthanum oxide in 150ml water, add 100ml 6N hydrochloric acid (3.2) and dilute to 1,000ml with water.
4. Apparatus
Atomic absorption spectrophotometer fitted with zinc lamp set at 213.8nm.
5. Procedure
5.1 Preparation of the solution for analysis.
Weigh to the nearest 0.001g, 5g of the prepared sample into a 250ml beaker, add 5ml hydrochloric acid (3.1) (take suitable precautions if there is a vigorous reaction due to carbon dioxide formation). If necessary add more hydrochloric acid (3.1), stirring until all effervescence has stopped. Evaporate the solution to dryness, occasionally stirring with a glass rod. Add 15ml 6N hydrochloric acid solution (3.2) and 120ml water. Stir with the glass rod, which should be left in the beaker, and cover with a watch glass. Boil the solution gently until dissolution appears complete and filter through a Whatman 541 (or equivalent) filter paper into a 250ml graduated flask. Wash the beaker and filter with 5ml of hot 6N hydrochloric acid solution (3.2) and with boiling water. Cool and make up to the mark with water. (The hydrochloric acid concentration of the solution should be about 0.5N.)
Dissolve the residue in 2ml hydrochloric acid (3.1), evaporate to dryness and add 5ml 6N hydrochloric acid solution (3.2). Heat, filter the solution into the 250ml graduated flask and make up to the mark with water. (The hydrochloric acid concentration of the solution should be about 0.5N.)
5.2 Blank test.
Carry out a blank determination repeating the procedure but omitting the sample.
5.3 Determination.
5.3.1 Preparation of the sample and blank test solutions.
Dilute the sample solutions prepared in 5.1 and the blank test solution (5.2) with 0.5N hydrochloric acid solution (3.3) to a concentration within the working range of the spectrophotometer (4). Each final solution must contain 10 % (V/V) of the lanthanum chloride solution (3.5).
5.3.2 Preparation of the calibration solutions.
By diluting the standard solution 3.4.1 with 0.5N hydrochloric acid solution (3.3) prepare at least 5 standard solutions of increasing concentrations corresponding to the optimal measuring range of the spectrophotometer. The final solutions must contain 10 % (V/V) lanthanum chloride solution (3.5).
5.4 Measurement.
Set up the spectrophotometer for the determination of zinc (213.8nm) using an oxidising air-acetylene flame. Spray successively, in triplicate, the standard solutions (5.3.2), the sample solutions and the blank test solutions (5.3.1), washing the instrument through with distilled water between each spraying. Plot the calibration curve, using the mean absorbances as the ordinates and the corresponding concentrations of zinc in μ/gml as the abscissae. Determine the concentration of the zinc in the final sample and blank solutions by reference to the calibration curves.
6. Expression of the results
Calculate the zinc content of the sample taking into account the weight of the test sample and the dilutions carried out in the course of the analysis. Express the result either as a percentage or as mg/kg.”
Regulation 4(3)
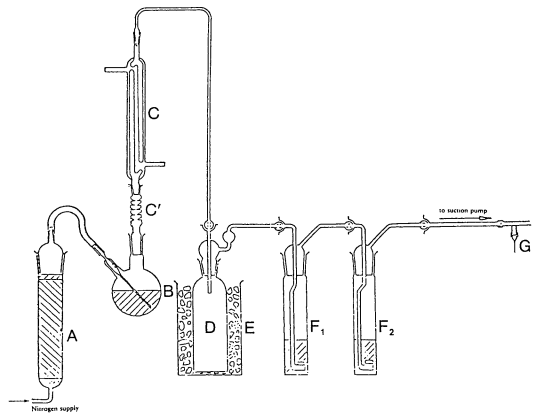
SCHEDULE 5DIAGRAM AND KEY TO BE INSERTED IN THE APPENDIX TO SCHEDULE 2 TO THE PRINCIPAL REGULATIONS
FIGURE 9
KEY TO FIGURE 9
A = Absorption tube filled with soda lime
B = Reaction flask
C' = Vigreux fractionating column 150 mm long
C = Double surface condenser 200 mm long
D = Drechsel bottle 250 ml
E = Ice bath
F1 and F2 = Absorption vessels 32 to 35mm diameter assembled with spherical ground joints Gas distributor with 10mm disc of low-porosity sintered glass
G = Suction-regulating device
Explanatory Note
(This note is not part of the Regulations)
These Regulations amend the Fertilisers (Sampling and Analysis) Regulations 1991 (“the principal Regulations”). Regulation 3(1) and (2) modifies the method prescribed by regulation 2 of the principal Regulations for determining the amount of material from which a sample may be taken for the purpose of analysis. Regulation 3(3) amends regulation 6(2) of the principal Regulations (methods of analysis) so that reference is made to the Fertilisers Regulations 1991 and in particular to section E of the Table in Schedule 1 to those Regulations. Regulation 4 amends Schedules 2 and 3 to the principal Regulations, in particular–
(a)in Part I of Schedule 2, by substituting for methods of analysis concerning magnesium a number of methods concerning calcium, magnesium, sodium, sulphur and sulphur in the form of sulphates;
(b)in Part II of Schedule 2, by omitting the method of analysis concerning magnesium and adding a method concerning zinc;
(c)by modifying the form of certificate in Schedule 3.
Regulation 4(1) and Schedules 1, 2 and 3, amending Part I of Schedule 2 to the principal Regulations, implement Commission Directive 89/519/EEC (OJ No. L265, 12.9.89, p.30) supplementing and amending Directive 77/535/EEC (OJ No. L213, 22.8.77, p.1) on the approximation of the laws of the Member States relating to methods of sampling and analysis of fertilisers.
1970 c. 40; section 74A was inserted in the Act by paragraph 6 of Schedule 4 to the European Communities Act 1972 (c. 68) and the Act was amended by the Agriculture Act 1970 Amendment Regulations 1982 (S.I. 1982/980). Section 66(1) contains definitions of the expressions “the Ministers”, “prescribed” and “regulations”. The definition of “the Ministers” was amended by the Transfer of Functions (Wales) (No. 1) Order 1978 (S.I. 1978/272), Schedule 5, paragraph 1.
Options/Help
Print Options
PrintThe Whole Instrument
Legislation is available in different versions:
Latest Available (revised):The latest available updated version of the legislation incorporating changes made by subsequent legislation and applied by our editorial team. Changes we have not yet applied to the text, can be found in the ‘Changes to Legislation’ area.
Original (As Enacted or Made): The original version of the legislation as it stood when it was enacted or made. No changes have been applied to the text.
More Resources
Access essential accompanying documents and information for this legislation item from this tab. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as enacted version that was used for the print copy
- lists of changes made by and/or affecting this legislation item
- confers power and blanket amendment details
- all formats of all associated documents
- correction slips
- links to related legislation and further information resources
More Resources
Use this menu to access essential accompanying documents and information for this legislation item. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as made version that was used for the print copy
- correction slips
Click 'View More' or select 'More Resources' tab for additional information including:
- lists of changes made by and/or affecting this legislation item
- confers power and blanket amendment details
- all formats of all associated documents
- links to related legislation and further information resources