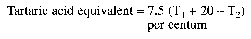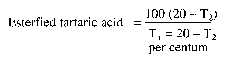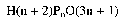Regulations 2(1) and 11(5)
SCHEDULE 5Purity Criteria
Each miscellaneous additive for which specific purity criteria are specified or referred to below shall not contain—
(a)more than 3 milligrams per kilogram of arsenic;
(b)more than 10 milligrams per kilogram of lead;
(c)more than 50 milligrams per kilogram of copper, or 25 milligrams per kilogram of zinc or 50 milligrams per kilogram of any combination of copper and zinc;
except in so far as those specific purity criteria provide otherwise or in the case of E 957 Thaumatin.
E 170(i). Calcium carbonate
| Description | Fine white microcrystalline or amorphous powder |
| Content | Not less than 97 per centum of CaCO3 on a volatile matter-free basis |
| Volatile matter | Not more than 1 per centum (determined by drying at 105°C to constant weight) |
| Matter insoluble in hydrochloric acid | Shall comply with the requirement for aluminium, iron, phosphate and matter insoluble in hydrochloric acid in the monograph for chalk in the British Pharmacopoeia 1973 at page 93 |
| Arsenic | Not more than 5 mg per kg. |
| Lead | Not more than 20 mg per kg. |
| Other inorganic impurities | Not more than 100 mg per kg of any of the following substances, namely antimony, copper, chromium, zinc or barium sulphate, or more than 200 mg per kg of any combination of those substances. |
E 200. Sorbic acid
E 202. Potassium sorbate
E 203. Calcium sorbate
E 210. Benzoic acid
E 211. Sodium benzoate
E 212. Potassium benzoate
E 213. Calcium benzoate
E 214. Ethyl p-hydroxybenzoate
| Synonyms | Ethyl 4-hydroxybenzoate
Ethyl ester of p-hydroxybenzoic acid
|
E 215. Sodium ethyl p-hydroxybenzoate
| Synonyms | Ethyl 4-hydroxybenzoate, sodium salt
Sodium ethyl para-hydroxybenzoate
|
E 216. Propyl p-hydroxybenzoate
| Synonyms | Propyl 4-hydroxybenzoate
Propyl para-hydroxybenzoate n-propyl p-hydroxybenzoate
|
E 217. Sodium propyl p-hydroxybenzoate
| Synonyms | Propyl 4-hydroxybenzoate, sodium salt
Sodium propyl para-hydroxybenzoate
Sodium n-propyl p-hydroxybenzoate
|
E 218. Methyl p-hydroxybenzoate
| Synonyms | Methyl 4-hydroxybenzoate
Methyl para-hydroxybenzoate
|
E 219. Sodium methyl p-hydroxybenzoate
| Synonyms | Methyl 4-hydroxybenzoate, sodium salt
Sodium methyl para-hydroxybenzoate
|
E 220. Sulphur dioxide
E 221. Sodium sulphite (anhydrous or heptahydrate)
E 222. Sodium hydrogen sulphite
| Synonym | Acid sodium sulphite
|
E 223. Sodium metabisulphite
E 224. Potassium metabisulphite
| The appropriate specific purity criteria contained in Council Directive 65/66/EEC as amended by Council Directive 67/428/EEC. |
E 226. Calcium sulphite
E 227. Calcium hydrogen sulphite
E 228. Potassium hydrogen sulphite
| Synonyms | Potassium bisulhite
Potassium acid sulphite
|
| The appropriate specific purity criteria contained in Council Directive 65/66/EEC as amended by Council Directive 86/604/EEC(). |
E 230. Biphenyl, diphenyl
E 231. Orthophenyl phenol
E 232. Sodium orthophenyl phenol
| Synonyms | Sodium biphenyl-2-yl-oxide
Sodium orthophenylphenate
|
E 233. Thiabendazole
| Synonyms | 2-(Thiazol-4-yl) benzimidazole
2-(4-thiazolyl) benzimidazole
|
| The appropriate specific purity criteria contained in Council Directive 65/66/EEC as amended by Council Directive 76/463/EEC. |
E 234. Nisin
| The criteria in the monograph for nisin contained in the Nutrition Meetings Report Series No. 45A (1969) of the United Nations' Food and Agriculture Organisation at page 53. |
E 239. Hexamethylene tetramine
E 249. Potassium nitrite
E 250. Sodium nitrite
E 251. Sodium nitrate
E 252. Potassium nitrate
E 260. Acetic acid
E 261. Potassium acetate
E 262(i). Sodium acetate
Sodium acetate, anhydrous
The criteria in the monograph for sodium acetate, anhydrous contained in the Food Chemicals Codex 1972 at page 718.
Sodium acetate
The criteria in the monograph for sodium acetate contained in the Food Chemicals Codex 1972 at page 717 except that the alkalinity shall be not more than 0.1 per centum (as sodium carbonate, Na2CO3).
In the case of:—
E 262(ii). Sodium diacetate
| Synonym | Sodium hydrogen diacetate |
E 263. Calcium acetate
E 270. Lactic acid
| The specific purity criteria for lactic acid contained in Council Directive 65/66/EEC. |
E 280. Propionic acid
E 281. Sodium propionate
E 282. Calcium propionate
E 283. Potassium propionate
| The appropriate specific purity criteria contained in Council Directive 65/66/EEC as amended by Council Directive 76/463/EEC. |
E 290. Carbon dioxide
| The specific purity criteria for carbon dioxide contained in Council Directive 65/66/EEC. Solid or liquid carbon dioxide shall be of equivalent purity to the gas. |
E 296. Malic acid
DL-Malic acidThe criteria in the monograph for malic acid contained in the Food Chemicals Codex 1972 at page 484 as amended by the Second Supplement to that Codex at page 27, except that the melting range shall be 130°C to 132°C (corrected) and that the method for determining the melting range shall be that specified or a method of equivalent accuracy.
L-Malic Acid |
| Description | White or nearly white crystalline powder or granules |
| Content Melting range | Not less than 99 per centum of C4H6O5. 99°C to 101°C. |
| Specific rotation [α] 20°C D | Not less than –2.4° and not more than –2.2° using a solution containing 8.5g L-malic and in 100 ml water. |
Malic acid
Fumaric acid
Residue on ignition
Water insoluble matter
| Shall comply with the limits given in the }monograph for malic acid in the Food Chemicals Codex 1972 at page 484. |
E 297. Fumaric acid
| The criteria in the monograph for fumaric acid contained in the Food Chemicals Codex 1972 at page 331. |
E 300. Ascorbic acid
E 301. Sodium ascorbate
E 302. Calcium ascorbate
E 304. Fatty acid esters of ascorbic acid
E 304(i). Ascorbyl palmitate
E 306. Tocopherol-rich extract
E 307. Alpha-tocopherol
E 308. Gamma-tocopherol
E 309. Delta-tocopherol
E 310. Propyl gallate
E 311. Octyl gallate
E 312. Dodecyl gallate
E 320. Butylated hydroxyanisole (BHA)
E 321. Butylated hydroxytoluene (BHT)
E 322. Lecithins
In the case of:—
E 325. Sodium lactate
E 326. Potassium lactate
E 327. Calcium lactate
E 330. Citric acid
E 331(i). Monosodium citrate
| Synonym | Sodium dihydrogen citrate |
E 331(ii). Disodium citrate
E 331(iii). Trisodium citrate
E 332(i). Monopotassium citrate
| Synonym | Potassium dihydrogen citrate |
E 332(ii). Tripotassium citrate
E 333(i). Monocalcium citrate
E 333(ii). Dicalcium citrate
E 333(iii). Tricalcium citrate
E 334. L-(+)-Tartaric acid
E 335(i). Monosodium L-(+)-tartrate
E 335(ii). Disodium L-(+)-tartrate
E 336(i). Monopotassium L-(+)-tartrate
E336(ii). Dipotassium L-(+)-tartrate
E 337. Sodium potassium L-(+)-tartrate
| Synonym | Potassium sodium tartrate |
E 338. Phosphoric acid
| Synonym | Orthophosphoric acid |
E 339(i). Monosodium phosphate
| Synonym | Monosodium orthophosphate |
E 339(ii). Disodium phosphate
| Synonym | Disodium orthophosphate Disodium hydrogen orthophosphate |
E 339(iii). Trisodium phosphate
| Synonym | Trisodium orthophosphate |
E 340(i). Monopotassium phosphate
| Synonyms | Monodipotassium orthophosphate Potassium dihydrogen orthophosphate |
E 340(ii). Dipotassium phosphate
| Synonyms | Dipotassium orthophosphate Dipotassium hydrogen orthophosphate |
E 340(iii). Tripotassium phosphate
| Synonym | Tripotassium orthophosphate |
E 341(i). Monocalcium phosphate
| Synonyms | Monocalcium orthophosphate Calcium tetrahydrogen diorthophosphate |
E 341(ii). Dicalcium phosphate
| Synonyms | Dicalcium orthophosphate Calcium hydrogen orthophosphate |
E 340(iii). Tricalcium phosphate
| Synonyms | Tricalcium orthophosphate Tricalcium diorthophosphate |
E 350(i). Sodium malate
| Description | Colourless or almost colourless aqueous solution. Sodium malate may be derived from either DL-malic acid or L-malic acid. |
| Content | Not less than 59.5 per centum of C4H4O5Na2. |
| Maleic acid | Not more than 0.05 per centum calculated on the C4H4O5Na2 content. |
E 350(ii). Sodium hydrogen malate
| Description | White odourless powder. Sodium hydrogen malate may be derived from either DL-malic acid or L-malic acid. |
| Content | Not less than 99 per centum of C4H5O5Na on a volatile matter-free basis. |
| Volatile matter | Not more than 2 per centum (determined by drying at 110°C for 3 hours) |
| Maleic acid | Not more than 0.05 per centum. |
E 351. Potassium malate
| Description | Colourless or almost colourless aqueous solution. Potassium malate may be derived from either DL-malic acid or L-malic acid. |
| Content | Not less than 59.5 per centum of C4H4O5K2 |
| Maleic acid | Not more than 0.05 per centum calculated on the C4H4O5K2 content. |
E 352(i). Calcium malate
| Description | White odourless powder. Calcium malate may be derived from either DL-malic acid or L-malic acid |
| Content | Not less than 97.5 per centum of C4H4O5Ca on a volatile matter-free basis. |
| Volatile matter | Not more than 2 per centum (determined by drying at 110°C for 3 hours) |
| Maleic acid | Not more than 0.05 per centum. |
| Fluoride | Not more than 30 mg per kg on a volatile matter-free basis |
E 352(ii). Calcium hydrogen malate
| Description | White odourless powder. Calcium hydrogen malate may be derived from either DL-malic acid or L-malic acid |
| Content | Not less than 97.5 per centum of (C4H5O5)2Ca on a volatile matter-free basis. |
| Volatile matter | Not more than 2 per centum (determined by drying at 110°C for 3 hours) |
| Maleic acid | Not more than 0.05 per centum. |
| Fluoride | Not more than 30 mg per kg on a volatile matter-free basis |
E 353. Metatartaric acid
| Description | White or yellow powder which consists chiefly of a mixture of polyesters obtained by the controlled dehydration of L-(+)-tartaric acid together with unchanged L-(+)-tartaric acid. |
| Specific absorption 1 per centum E 1 cm | Not more than 1.5 × 10−2at 430 nm. (determined using a filtered aqueous solution). |
| Identification | Place 5 to 10 mg of sample in a test tube. Add 2 ml sulphuric acid (about 94 per centum H2SO4) plus two drops of resorcinol reagent (2 g. resorcinol dissolved in 100 ml water plus 0.5 ml sulphuric acid) and heat to 150°C. An intense violet colour is produced. |
| Content | Not less than the equivalent of 105 per centum of tartaric acid (C4H6O6). The esterified tartaric acid content shall be not less than 27 per centum and not more than 38 per centum of the tartaric acid equivalent when determined by the following method:
Add three drops of bromothymol blue indicator (0.04 per centum weight/volume solution of bromothymol blue in 95 per centum volume/volume ethanol) to 50 ml of freshly prepared 2 per centum weight/volume cold aqueous solution of metatartaric acid. Titrate with N aqueous sodium hydroxide solution to a blue-green colour (T1ml.). Add a further 20 ml of N aqueous sodium hydroxide solution and leave for 2 hours at room temperature.
Titrate with N aqueous sulphuric acid solution (T2ml).
Calculations:
|
| Specific rotation [α] 20°C D | Not less than + 12.5° and not more than + 13.5° (using a filtered 10 per centum weight/volume aqueous solution). |
| Matter insoluble in water (at about 20°C) | Not more than 2.5 per centum (insoluble matter weighed after drying for 3 hours at 70°C in a vacuum oven). |
| Pyruvic acid | Not more than 0.5 per centum. |
E 355. Adipic acid
| The criteria in the monograph for adipic acid contained in the Food Chemicals Codex 1972 at page 21. |
E 363. Succinic acid
| The criteria in the monograph for succinic acid contained in the Food Chemicals Codex 1972 at page 800. |
E 380. Triammonium citrate
| Synonym | Ammonium citrate |
| The criteria in the monograph for ammonium citrate contained in the British Pharmaceutical Codex 1973 at page 830. |
E 385. Calcium disodium ethylenediamine — N N N'N' — tetra-acetate
| Synonym | Sodium calciumedate |
| The criteria in the monograph for sodium calciumedetate contained in the British Pharmacopoeia 1973 at page 425. |
E 400. Alginic acid
E 401. Sodium alginate
E 402. Potassium alginate
E 403. Ammonium alginate
E 404. Calcium alginate
E 405. Propane-1,2-diol alginate
| Synonym | Propylene glycol alginate |
E 406. Agar
| The specific purity criteria for agar contained in Council Directive 78/663/EEC. |
E 407. Carrageenan
| The specific purity criteria for carrageenan contained in Council Directive 78/663/EEC, as amended by Article 1 of Commission Directive 90/612/EEC(). |
E 410. Locust bean gum
E 412. Guar gum
E 413. Tragacanth
E 414. Acacia
E 415. Xanthan gum
| The specific purity criteria for xanthan gum contained in Council Directive 78/663/EEC, as amended by Article 1.2(b) of Council Directive 82/504/EEC. |
E 416. Karaya gum
| Synonym | Sterculia gum |
| The criteria in the monograph for karaya gum contained in the Food Chemicals Codex 1981 at page 157. |
E 420(i). Sorbitol
E 420(ii). Sorbitol syrup
E 421. Mannitol
E 422. Glycerol
| As set out in the Annex to Council Directive 78/663/EEC. |
E 432. Polyoxyethylene (20) sorbitan monolaurate
| Synonym | Polysorbate 20 |
| The criteria in the monograph for polysorbate 20 contained in the Food Chemicals Codex 1981 at page 234. |
E 433. Polyoxyethylene (20) sorbitan monooleate
| Synonym | Polysorbate 80 |
| The criteria in the monograph for polysorbate 80 contained in the Food Chemicals Codex 1981 at page 236 except that the final sentence of the description (requirement to conform to the regulations of the federal Food and Drug Administration pertaining to specifications for fats or fatty acids derived from edible sources) shall be deleted. |
E 434. Polyoxyethylene (20) sorbitan monopalmitate
| Synonym | Polysorbate 40 |
| The criteria in the monograph for polyoxyethylene (20) sorbitan monopalmitate contained in the Food and Nutrition Paper No. 4 (1978) of the United Nations' Food and Agriculture Organisation at page 278. |
E 435. Polyoxyethylene (20) sorbitan monostearate
| Synonym | Polysorbate 60 |
| The criteria in the monograph for polysorbate 60 contained in the Food Chemicals Codex 1981 at page 235 except that the final sentence of the description (requirement to conform to the regulations of the federal Food and Drug Administration pertaining to specifications for fats or fatty acids derived from edible sources) shall be deleted. |
E 436. Polyoxyethylene (20) sorbitan tristearate
| Synonym | Polysorbate 65 |
| The criteria in the monograph for polysorbate 65 contained in the Food Chemicals Codex 1981 at page 235 except that the final sentence of the description (requirement to conform to the regulations of the federal Food and Drug Administration pertaining to specifications for fats or fatty acids derived from edible sources) shall be deleted. |
E 440(i). Pectin
E 440(ii). Amidated pectin
E 442. Ammonium phosphatides
| Description | Ammonium phosphatides exist as an unctuous semi-solid (at 25°C). They consist essentially of a mixture of the ammonium salts of phosphatidic acids derived from partially hardened rapeseed oil together with unreacted partially hardened rape-seed oil. |
| Matter insoluble in petroleum ether (40°C-60°C) | Total: Not more than 2.5 per centum. Inorganic matter: not more than 0.2 per centum. |
| pH of an aqueous extract of melted ammonium phosphatides | Not less than 6.0 and not more than 8.0 |
| Phosphorus | Not less than 3.0 per centum and not more than 3.4 per centum. |
| Ammonium nitrogen | Not less than 1.2 per centum and not more than 1.5 per centum. |
| Arsenic | Not more than 5 mg per kg. |
E 450(i). Disodium diphosphate
E 450(ii). Trisodium diphosphate
E 450(iii). Tetrasodium diphosphate
E 450(v). Tetrapotassium diphosphate
E 450(vi). Dicalcium diphosphate
| Synonyms | Dicalcium pyrophosphate Calcium pyrophosphate |
| The criteria in the monograph for calcium pyrophosphate contained in the Food Chemicals Codex 1972 at page 153. |
E 451(i). Pentasodium triphosphate
E 451(ii). Pentapotassium triphosphate
E 452(i). Sodium polyphosphate
E 452(ii). Potassium polyphosphate
E 452(iv). Calcium polyphosphates
| Description | Calcium polyphosphates exist as a fine white powder or crystals or colourless glassy platelets. They are reproducible heterogeneous mixtures of calcium salts of condensed polyphosphoric acids of general formula:
|
| Content (expressed as P2O5) | Not less than 50 per centum and not more 71 per centum of an anhydrous basis. |
| pH (1 per centum aqueous solution) | For water soluble phosphates only: not less than 4.0 and not more than 9.0. |
| Cyclic phosphate | Not more than 8 per centum calculated on the P2O5 content. |
| Fluoride | Not more than 15 mg per kg calculated on the P2O5 content. |
E 460(i). Microcrystalline cellulose
| The specific purity criteria for microcrystalline cellulose contained in Council Directive 78/663/EEC, as amended by Article 1.2(c) of Council Directive 82/504/EEC. |
E 460(ii). Powdered cellulose
| Synonym | Alpha-cellulose |
| The criteria in the monograph for cellulose, powdered, contained in the Food Chemicals Codex 1981 at page 80. Additionally the level of lead present shall not exceed 1 mg per kg. |
E 461. Methylcellulose
E 463. Hydroxypropylcellulose
E 464. Hydroxypropylmethylcellulose
E 465. Ethylmethylcellulose
| Synonym | Methylethylcellulose |
E 466. Carboxymethylcellulose
| Synonym | Sodium carboyxmethylcellulose |
| The specific purity criteria for carboxymethylcellulose contained in Council Directive 78/663/EEC, as amended by Article 1 of Commission Directive 90/612/EEC. |
E 470a. Sodium, potassium and calcium salts of fatty acids
E 471. Mono- and diglycerides of fatty acids
E 472(a). Acetic acid esters of mono- and diglycerides of fatty acids
| Synonym | Acetylated mono- and diglycerides |
E 472(b). Lactic acid esters of mono- and diglycerides of fatty acids
| Synonyms | Lactylated mono- and diglycerides
Lactoglycerides
|
E 472(c). Citric acid esters of mono- and diglycerides of fatty acids
E 472(d). Tartaric acid esters of mono- and diglycerides of fatty acids
E 472(e). Mono-and diacetyl tartaric acid esters of mono- and diglycerides of fatty acids
| Synonym | Mono- and diacetyl tartaric acid esters of mono-and diglycerides |
E 472(f). Mixed acetic and tartaric acid esters of mono- and diglycerides of fatty acids
E 473. Sucrose esters of fatty acids
| The specific purity criteria for sucrose esters of fatty acids contained in Council Directive 78/663/EEC, as amended by Article 1 of Commission Directive 90/612/EEC and Article 1 of Commission Directive 92/4/EEC(). |
E 474. Sucroglycerides
| The specific purity criteria for sucroglycerides contained in Council Directive 78/663/EEC, as amended by Article 1.2(e) of Council Directive 82/504/EEC. |
E 475. Polyglycerol esters of fatty acids
| The specific purity criteria for polyglycerol esters of non-polymerised fatty acids contained in Council Directive 78/663/EEC. |
E 476. Polyglycerol polyricinoleate
| Synonym of castor oil. | Polyglycerol esters of polycondensed fatty acids |
| Description | The polyglycerol esters of polycondensed fatty acids of castor oil exist as a highly viscous liquid (at 25°C). They are essentially a complex mixture of the partial esters and ethers of polyglycerol with linearly interesterified (polycondensed) fatty acids derived from castor oil. The polycondensed castor oil fatty acids are prepared by condensation in the absence of oxygen and have an average of about 5 fatty acid residues per molecule. The polyglycerol moiety is predominantly di-, tri- and tetra-glycerol and contains not more than 10 per centum of polyglycerols equal to or higher than heptaglycerol. |
| Refractive index, m65D°C | Not less than 1.4630 and not more than 1.4665. |
| Hydroxyl value | Not less than 80 and not more than 100. |
| Iodine value | Not less than 72 and not more than 103 (Wijs). |
| Acid value | Not more than 6 mg KOH per g. |
E 477. Propane-1,2-diol esters of fatty acids
| Synonym | Propylene glycol esters of fatty acids. |
| The specific purity criteria for propane-1,2-diol esters of fatty acids contained in Council Directive 78/663/EEC, as amended by Article 1.2(f) of Council Directive 82/504/EEC. |
E 481. Sodium stearoyl-2-lactylate
E 482. Calcium stearoyl-2-lactylate
E 483. Stearyl tartrate
E 491. Sorbitan monostearate
| The criteria in the monograph for sorbitan monostearate contained in the Food Chemicals Codex 1981 at page 307 except that the final sentence of the description (requirement to conform to the regulations of the federal Food and Drug Administration pertaining to specifications for fats or fatty acids derived from edible sources) shall be deleted. |
E 492. Sorbitan tristearate
| The criteria in the monograph for sorbitan tristearate contained in the Food and Nutrition Paper No. 4 (1978) of the United Nations' Food and Agriculture Organisation at page 297. |
E 493. Sorbitan monolaurate
| The criteria in the monograph for sorbitan monolaurate contained in the British Pharmaceutical Codex 1973 at page 465. |
E 494. Sorbitan monooleate
| The criteria in the monograph for sorbitan monooleate contained in the British Pharmaceutical Codex 1973 at page 466. |
E 495. Sorbitan monopalmitate
| The criteria in the monograph for sorbitan monopalmitate contained in the Food and Nutrition Paper No. 4 (1978) of the United Nations' Food and Agriculture Organisation at page 293. |
E 500(i). Sodium carbonate
| Description | Colourless crystals or white granular or crystalline powder. The anhydrous salt is hygroscopic and the decahydrate is efflorescent. |
| Content | Not less than 98 per centum of Na2CO2 on a volatile matter-free basis. |
| Volatile matter | Not more than:
2 per centum for the non-hydrated substance;
15 per centum for the monohydrate;
65 per centum for the decahydrate; (determined by the method for loss on drying in the monograph for sodium carbonate in the Food Chemicals Codex 1972 at page 731.)
|
| Matter insoluble in dilute ammonia solution | |
| Sulphate | Not more than 0.4 per centum on a volatile matter-free basis. |
| Chloride | Not more than 0.4 per centum on a volatile matter-free basis |
| Iron | Not more than 40 mg per kg on a volatile matter-free basis. |
E 500(ii). Sodium hydrogen carbonate
| Synonym | Sodium bicarbonate |
| The criteria in the monograph for sodium bicarbonate contained in the Food Chemicals Codex 1972 at page 727. |
E 500(iii). Sodium sesquicarbonate
| The criteria in the monograph for sodium sesqicarbonate contained in the Food Chemicals Codex 1972 at page 765. |
E 501(i). Potassium carbonate
| Description | |
| Content | Not less than 98 per centum K2CO3 on a volatile matter-free basis. |
| Volatile matter | |
E 501(ii). Potassium hydrogen carbonate
| Synonym | Potassium bicarbonate |
| The criteria in the monograph for potassium bicarbonate contained in the Food Chemicals Codex 1972 at page 642. |
E 503(i). Ammonium carbonate
| The criteria in the monograph for ammonium carbonate contained in the Food Chemicals Codex 1972 at page 45. |
E 503(ii). Ammonium hydrogen carbonate
| Synonym | Ammonium bicarbonate |
| The criteria in the monograph for ammonium bicarbonate contained in the Food Chemicals Codex 1972 at page 44. |
E 504. Magnesium carbonates
Magnesium carbonate, heavy
| The criteria in the monograph for heavy magnesium carbonate contained in the European Pharmacopoeia Vol. 1, 1969 at page 322. |
Magnesium carbonate, light
| The criteria in the monograph for light magnesium carbonate contained in the European Pharmacopoeia Vol. 1, 1969 at page 321. |
E 507. Hydrochloric acid
| The criteria in the monograph for concentrated hydrochloric acid contained in the European Pharmacopoeia Vol. II, 1971 at page 145. |
E 508. Potassium chloride
| The criteria in the monograph for potassium chloride contained in the Food Chemicals Codex 1972 at page 646. |
E 509. Calcium chloride
Calcium chloride, anhydrous
| The criteria in the monograph for calcium chloride, anhydrous contained in the Food Chemicals Codex 1972 at page 124. |
Calcium chloride
| Description | The dihydrate consists of deliquescent white odourless fragments or granules. The hexahydrate consists of deliquescent colourless and odourless crystals. |
| Content | |
| Magnesium and alkali salts | Not more than 2 per centum, determined by the method in the monograph for calcium chloride contained in the Food Chemicals Codex 1972 at page 123 except that the weight of the residue shall not exceed 10 mg. |
| Fluoride | Not more than 40 mg per kg on an anhydrous basis. |
E 513. Sulphuric acid
| The criteria in the monograph for sulphuric acid contained in the Food Chemicals Codex 1972 at page 802. |
E 514(i). Sodium sulphate
| The criteria in the monograph for sodium sulphate contained in the Food Chemicals Codex 1972 at page 775. |
E 515(i). Potassium sulphate
| The criteria in the monograph for potassium sulphate contained in the Food Chemicals Codex 1972 at page 670. |
E 516. Calcium sulphate
| The criteria in the monograph for calcium sulphate contained in the Food Chemicals Codex 1972 at page 163. |
E 522. Aluminium potassium sulphate
| Synonyms | Potassium aluminium sulphate Potash alum. |
| The criteria in the monograph for alum contained in the European Pharmacopoeia Vol. 1, 1969 at page 243. |
E 524. Sodium hydroxide
| The criteria in the monograph for sodium hydroxide contained in the Food Chemicals Codex 1972 at page 743. |
E 525. Potassium hydroxide
| The criteria in the monograph for potassium hydroxide contained in the Food Chemicals Codex 1972 at page 652. |
E 526. Calcium hydroxide
| Description | Soft white powder. |
| Solubility | 1 g dissolves in 630 ml of water at 25°C, and in 1300 ml of boiling water. Soluble in glycerol and in a saturated solution of sucrose. Insoluble in ethanol. |
| Content | Not less than 92 per centum of Ca(OH)2. |
| Matter insoluble in dilute Hydrochloric acid (about 10 per centum weight/ volume HCL) | Not more than 0.5 per centum. |
| Magnesium and alkali salts | Not more than 6 per centum, determined by the method in the monograph for calcium hydroxide contained in the Food Chemicals Codex 1972 at page 131 except that the weight of the residue shall not exceed 15 mg. |
| Carbonate | When 2 g of calcium hydroxide is mixed with 50 ml of water and an excess of dilute hydrochloric acid (approximately 2N) is added, no more than a slight effervescence is produced. |
| Sulphate | Not more than 0.35 per centum. |
| Fluoride | Not more than 50 mg per kg. |
E 527. Ammonium hydroxide
| The criteria in the monograph for ammonium hydroxide contained in the Food Chemicals Codex 1972 at page 48. |
E 528. Magnesium hydroxide
| The criteria in the monograph for magnesium hydroxide contained in the British Pharmaceutical Codex 1973 at page 277. |
E 529. Calcium oxide
| The criteria in the monograph for calcium hydroxide contained in the Food Chemicals Codex 1972 at page 138. |
E 530. Magnesium oxide
Magnesium oxide, heavy
| Description | White fine odourless powder. |
| Solubility | Practically insoluble in water.
Soluble in dilute acids with, at most, slight effervescence.
|
| Apparent volume | 20 g of heavy magnesium oxide occupies a volume of about 50 ml. |
| Content | Not less than 98 per centum of MgO calculated with reference to the ignited substance and determined by the assay method contained in the monograph for light magnesium oxide in the European Pharmacopoeia Vol. I, 1969 at page 319. |
| Loss on ignition | Not more than 5 per centum (determined by ignition at 900°C to 950°C to constant weight). |
| Matter soluble in water | Not more than 2 per centum, determined by the method for soluble substances contained in the monograph for light magnesium oxide in the European Pharmacopoeia Vol. I, 1969 at page 319. |
| Matter insoluble in acetic acid | Not more than 0.1 per centum when determined by the following method:
Dissolve 5 g heavy magnesium oxide in a mixture of 70 ml acetic acid (see Note 1) and 30 ml water. Heat to boiling for 2 minutes, cool and dilute to 100 ml with dilute acetic acid (see Note 2). Filter through a sintered glass filter. Any residue, after washing with water, drying and ignition at 600°C, shall weigh not more than 5 mg.
|
| Sulphate | Not more than 0.75 per centum. |
| Chloride | Not more than 0.07 per centum. |
| Calcium | Not more than 2 per centum. |
| Iron | Not more than 0.1 per centum. |
| Arsenic | Not more than 4 mg per kg. |
| Heavy metals | Not more than 40 mg per kg. |
Note 1:
Acetic acid: contains not less than 29 per centum weight/volume and not more than 31 per centum weight/volume of C2H4O2. Dilute 30 g glacial acetic acid (98 per centum weight/volume C2H4O2) to 100 ml with water.
Note 2:
Dilute acetic acid: contains not less than 11.5 per centum weight/volume and not more than 12.5 per centum weight/volume of C2H4O2. Dilute 12 g or 11.7 ml glacial acetic acid (98 per centum weight/volume C2H4O2) to 100 ml with water and, if necessary, adjust the concentration of the solution.
Magnesium oxide, light
| The criteria in the monograph for light magnesium oxide contained in the European Pharmacopoeia Vol I, 1969 at page 319. |
E 535. Sodium ferrocyanide
| Synonyms | Sodium hexacyanoferrate (II) |
| The criteria in the monograph for sodium ferrocyanide contained in the Food Chemicals Codex 1972 at page 741. |
E 536. Potassium ferrocyanide
| Synonym | Potassium hexacyanoferrate (II) |
| Description | Odourless lemon yellow crystals. |
| Solubility | Soluble in water and in acetone. Insoluble in ethanol, in ether and in hydrocarbons. |
| Content | Not less than 98 per centum of K4Fe(CN)6. 3H2O. |
| Free moisture | Not more than 1 per centum (determined by the method for free moisture in the monograph for sodium ferrocyanide in the Food Chemicals Codex 1972 at page 741). |
| Chloride | Not more than 0.1 per centum. |
| Sulphate | Not more than 0.1 per centum. |
E 541. Sodium aluminium phosphate, acidic
| The criteria in the monograph for sodium aluminium phosphate, acidic contained in the Food Chemicals Codex 1972 at page 722. |
E 551. Silicon dioxide
| Synonym | Silica, chemically prepared. |
| Description | Silica aerogel is a whie fluffy powdered or granular microcellular silica. Hydrated silica is a precipitated hydrated silicon dioxide occurring as a fine white amorphous powder or as beads or granules. |
| Content | |
| Volatile matter | Hydrated silica: not more than 7 per centum (determined by drying at 105°C for 2 hours). |
| Loss on ignition | Not more than 13 per centum (determined by ignition at 1000°C to constant weight). |
| Soluble ionisable salts (expressed as Na2SO4) | Not more than 5 per centum. |
E 552. Calcium silicate
| Description | White to off-white free-flowing powder. |
| Solubility | |
| Content: | |
| (expressed as SiO2) | Not less than 72 per centum and not more than 78 per centum on a volatile matter-free basis. |
| (expressed as CaO) | Not less than 16 per centum and not more than 21 per centum on a volatile matter-free basis. |
| (expressed as Na2O) | Not less than 2 per centum and not more than 4 per centum on a volatile matter-free basis. |
| Volatile matter | Not more than 6 per centum (determined by drying at 105°C for 2 hours). |
| Loss on ignition | Not less than 7 per centum and not more than 14 per centum (determined by ignition at 1000°C to constant weight). |
E 553a(i). Magnesium silicate
| The criteria in the monograph for magnesium silicate contained in the Food Chemicals Codex 1972 at page 479. |
E 553a(ii). Magnesium trisilicate
| The criteria in the monograph for magnesium trisilicate contained in the British Pharmacopoeia 1973 at page 276. |
E 553b. Talc
| Description | Talc is a native hydrous magnesium silicate sometimes containing a small proportion of aluminium silicate |
| It shall comply with the requirements for appearance, characteristics and limits of impurities in the monograph for magnesium silicate contained in the Nutrition Meetings Report Series 46B 1970 of the Food and Agriculture Organisation of the United Nations at page 114. The amount of material soluble in dilute hydrochloric acid shall be not more than 2 per centum and the amount of water soluble substances shall be not more than 0.2 per centum. |
E 554. Sodium aluminium silicate
| Synonyms | |
| Description | Fine white amorphous powder or beads. |
| Content: | |
| (expressed as SiO2) | Not less than 70 per centum and not more than 80 per centum on a volatile matter-free basis. |
| (expressed as Al2O3) | Not less than 8 per centum and not more than 11 per centum on a volatile matter-free basis. |
| (expressed as Na2O) | Not less than 5 per centum and not more than 10 per centum on a volatile matter-free basis. |
| Volatile matter | Not more than 8 per centum (determined by drying at 105°C for 2 hours) |
| Loss on ignition | Not less than 10 per centum and not more than 14 per centum (determined by ignition at 1000°C to constant weight). |
E 556. Calcium aluminium silicate
| Synonyms | |
| Description | Fine white free-flowing powder. |
| Content: | |
| (expressed as SiO2) | Not less than 44 per centum and not more than 50 per centum on a volatile matter-free basis. |
| (expressed as Al2O3) | Not less than 3 per centum and not more than 5 per centum on a volatile matter-free basis. |
| (expressed as CaO) | Not less than 32 per centum and not more than 38 per centum on a volatile matter-free basis. |
| (expressed as Na2O) | Not less than 0.5 per centum and not more than 4 per centum on a volatile matter-free basis. |
| Volatile matter | Not more than 10 per centum (determined by drying at 105°C for 2 hours). |
| Loss on ignition | Not less than 14 per centum and not more than 18 per centum (determined by ignition at 1000°C to constant weight). |
E 559. Aluminium silicate (Kaolin)
Kaolin, heavy
| The criteria in the monograph for heavy kaolin contained in the British Pharmaccopoeia 1968 at page 538 as amended by the 1969 Addendum at page 54. |
Kaolin, light
| The criteria in the monograph for light kaolin contained in the British Pharmacopoeia 1968 at page 539 as amended by the 1969 Addendum at page 54. |
E 575. Glucono-delta-lactone
| Synonym | D-Glucono-l,5-lactone |
| The criteria in the monograph for glucono delta-lactone contained in the Food Chemicals Codex 1972 at page 346. |
E 576. Sodium gluconate
| The criteria in the monograph for sodium gluconate contained in the Food Chemicals Codex 1972 at page 742. |
E 577. Potassium gluconate
| Description | White free-flowing powder. |
| Solubility | Freely soluble in water. Practically insoluble in ethanol and in ether. |
| Content | Not less than 97 per centum of C6H11O7K on a volatile matter-free basis. |
| Volatile matter | Not more than 3 per centum (determined by drying in a vacuum at 105°C for 4 hours). |
| Reducing substances (expressed as glucose) | Not more than 0.5 per centum. |
E 578. Calcium gluconate
| The criteria in the monograph for calcium gluconate contained in the Food Chemicals Codex 1972 at page 129. |
E 621. Monosodium glutamate
| Synonyms | |
| Formula | C5H8NNaO4.H2O (molecular weight 187.13). |
| The criteria in the monograph for monosodium L-glutamate contained in the Food Chemicals Codex 1981 at page 203. |
E 627. Disodium guanylate
| Synonyms | |
| Formula | C10H12N5Na2O8P.xH2O (molecular weight (anhydrous) 407.20). |
| The criteria in the monograph for disodium guanylate contained in the Food Chemicals Codex 1981 at page 105. |
E 631. Disodium inosinate
| Synonyms | |
| Formula | C10H11N4Na2O8P.xH2O (molecular weight (anhydrous) 392.19). |
| The criteria in the monograph for disodium inosinate contained in the Food Chemicals Codex 1981 at page 106. |
E 635. Disodium 5' -ribonucleotides
| Description | White or nearly white crystalline powder consisting of a mixture of guanosine 5' -(disodium phosphate) and inosine 5' -(disodium phosphate) in approximately equal proportions.
Soluble in water, practically insoluble in ethanol.
|
| Content | Not less than 97% and not more than 102% of C10H12N5Na2O8P and C10H11N4Na2O8P, and not less than 47% and not more than 53% of C10H12N5Na2O8P or of C10H11N4Na2O8P, in every case calculated on an anhydrous basis. |
| Moisture | Not less than 22% and not more than 26% (Karl Fischer). |
| pH (5% aqueous solution) | Not less than 7.0 and not more than 8.5. |
| Ammonium salts | Place 100 mg of sample in a test tube.
Add 50 mg magnesium oxide plus 1 ml of water.
Heat on a water bath for 5 minutes; the vapour evolved does not affect the colour of moist litmus paper.
|
| Amino acids | Place 5 ml of a 0.1% (weight/volume) solution in a test tube. Add 1 ml of a 2% (weight/volume) solution of ninhydrin and heat for 3 minutes; no blue colour is produced. |
| Other nucleotides | The paper chromatogram obtained when sodium 5' -ribonucleotide is analysed using the procedure described for “other nucleotides” in the monograph for disodium guanylate contained in the Food Chemicals Codex 1981 at page 105 shall show no spots other than those for guanosine 5' -(disodium phosphate) and inosine 5' -(disodium phosphate). |
E 640. Glycine
| The criteria in the monograph for glycine contained in the Food Chemicals Codex 1972 at page 359. |
E 900. Dimethylpolysiloxane
| Synonym | Dimethyl silicone. |
| Appearance | Clear colourless odourless liquid free from extraneous matter. |
| Solubility | |
| Volatile matter | Not more than 2 per centum (determined by drying at 200°C for 4 hours). |
| Identification | Shall comply with the identification tests in the monograph for dimethicone in the British Pharmaceutical Codex 1973 at page 168. |
| Acidity | Shall comply with the requirement for acidity in the monograph for dimethicone in the British Pharmaceutical Codex 1973 at page 168. |
| Total silicon | Not less than 37.3 and not more than 38.5 per centum. |
| Refractive index n 25°C D | Not less than 1.400 and not more than 1.405. |
| Viscosity (25°C) | Not less than 300 and not more than 1050 centistokes. |
| Relative density d 20°C 4°C | Not less than 0.960 and not more than 0.980. |
E 901. Beeswax, white and yellow
Beeswax, white
| The criteria in the monograph for beeswax, white contained in the Food Chemicals Codex 1972 at page 75, except that the ester value shall be not less than 70 and not more than 80. |
Beeswax, yellow
| The criteria in the monograph for beeswax, yellow contained in the Food Chemicals Codex 1972 at page 77, except that the ester value shall be not less than 70 and not more than 80 |
E 903. Carnauba wax
| The criteria in the monograph for carnauba wax contained in the Food Chemicals Codex 1972 at page 170. |
E 904. Shellac
| The standard for machine-made shellac contained in British Standard 3722:1964. |
E 941. Nitrogen
| The standard for nitrogen type 2 contained in British Standard 4366:1968. |
E 942. Nitrous oxide
| The criteria in the monograph for nitrous oxide contained in the European Pharmacopoeia Vol. II 1971 at page 316. |
E 948. Oxygen
| The criteria in the monograph for oxygen contained in the European Pharmacopoeia Vol. II 1971 at page 328. |
E 950. Acesulfame potassium
E 951. Aspartame
E 953. Isomalt
E 957. Thaumatin
E 959. Neohesperidine DC
E 965(i). Maltitol
E 965(ii). Maltitol syrup
E 966. Lactitol
Xylitol
E 999. Extract of Quillaia
| The aqueous extract of the product complying with the monograph for Quillaia or for powdered Quillaia, in each case, contained in the British Pharmacopoeia 1980, at page 382. |
E 1200. Polydextrose
| Description | Polydextrose is an off-white to light tan coloured, water-soluble powder. It consists of a randomly bonded condensation polymer produced by the reaction of D-glucose with sorbitol and citric acid. Free acid groups may be neutralised with potassium hydroxide. |
| Content | Not less than 90% of polymer on an ash-free and water-free basis. |
| Free glucose | Not more than 4% of an ash-free and water-free basis. |
| Free 1,6 anhydro-D-glucose | Not more than 4% on an ash-free and water-free basis. |
| Free sorbital | Not more than 2% on an ash-free and water-free basis. |
| Water | Not more than 4% (Karl Fischer). |
| pH (10% aqueous solution) | Not less than 2.5 and not more than 3.5 (not less than 5.0 and not more than 6.0 for the neutralised product). |
| Sulphated ash | Not more than 0.3% (not more than 3.0% for the neutralised product). |
| Arsenic | Not more than 1 mg/kg. |
| Lead | Not more than 1 mg/kg. |
Propane-1,2-diol (propylene glycol)
| As set out in the Annex to Council Directive 78/663/EEC. |